Alkenes
General formula: CnH2n • Functional group: C=C double bond
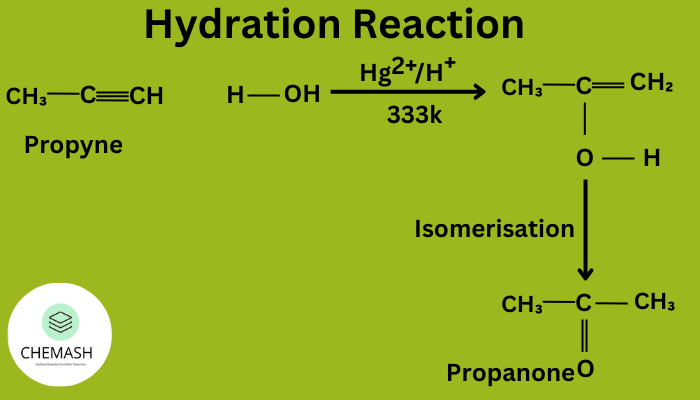
Table of Contents
- Introduction
- Physical Properties
- Chemical Properties (Key Reactions)
- Methods of Preparation
- Isomerism in Alkenes
- Uses of Alkenes
- Alkenes Quiz
- References & Related Links
Introduction
Alkenes are unsaturated hydrocarbons containing at least one carbon–carbon double bond (C=C). They follow the formula CnH2n, having two fewer hydrogens than the corresponding alkanes. The C=C bond imparts greater reactivity compared with alkanes.
Physical Properties
- Generally colorless gases or liquids at room temperature.
- Nonpolar; insoluble in water but soluble in organic solvents (e.g., benzene, ether).
- Boiling points increase with molecular mass due to stronger dispersion forces.
Chemical Properties (Key Reactions)
The C=C bond undergoes electrophilic addition and other transformations:
- Addition of Halogens: Br2/Cl2 → vicinal dihalides.
- Addition of HX: HCl/HBr/HI adds across C=C (Markovnikov’s rule).
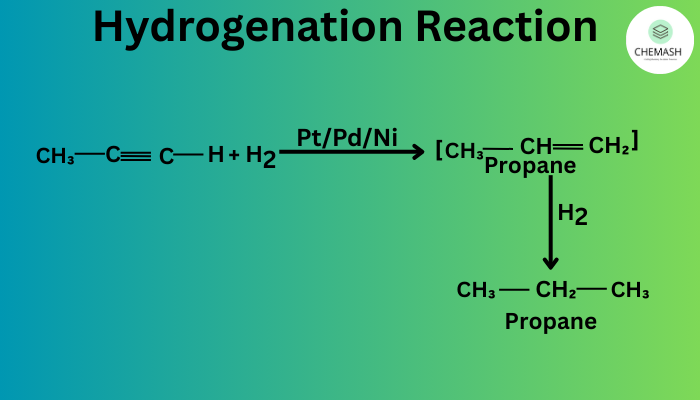
- Hydrogenation: H2 with Pt/Pd/Ni → corresponding alkanes.
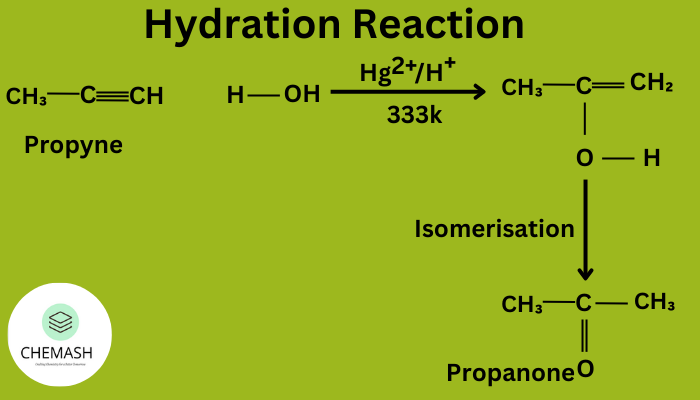
- Hydration: Acid-catalyzed addition of water → alcohols.
- Polymerization: Chain growth to polymers (e.g., polyethylene).
Methods of Preparation
- Dehydration of Alcohols: Heat with conc. H2SO4 or alumina.
Example: C2H5OH → C2H4 + H2O - Dehydrohalogenation of Alkyl Halides: Alcoholic KOH eliminates HX.
Example: C2H5Br + KOH → C2H4 + KBr + H2O - Cracking of Petroleum Fractions: Thermal/catalytic cleavage of higher alkanes.
- Partial Dehydrogenation of Alkanes: Catalytic removal of H2 at high temperature.
- From Alkynes: Lindlar-catalyzed partial hydrogenation → cis-alkenes.
Isomerism in Alkenes
- Cis: similar groups on the same side of the double bond.
- Trans: similar groups on opposite sides.
Restricted rotation around C=C leads to geometric (cis–trans or E/Z) isomerism when each double-bond carbon bears two different groups.
Uses of Alkenes
- Feedstocks for plastics, alcohols, epoxy compounds, and detergents.
- Ethene is used for artificial fruit ripening.
- Polymerization yields materials such as polyethylene and polypropylene for packaging and manufacturing.
Alkenes Quiz
- What is the general formula of alkenes?
Answer: CnH2n
Because one double bond reduces the hydrogen count by two vs. alkanes. - Which reagent converts an alkyl halide to an alkene?
Answer: Alcoholic KOH (dehydrohalogenation). - What type of isomerism is typical for alkenes?
Answer: Geometric (cis–trans/E–Z). - Which catalysts hydrogenate alkenes?
Answer: Pt, Pd or Ni. - Name the reaction where alcohols lose water to form alkenes.
Answer: Dehydration of alcohols. - Product of ethene + Br2 (in CCl4)?
Answer: 1,2-dibromoethane. - Polymer formed from ethene?
Answer: Polyethylene.
Summary: Alkenes (CnH2n) are reactive unsaturated hydrocarbons. Their C=C bond drives hallmark additions (HX, X2, H2, H2O), multiple preparation routes, geometric isomerism, and wide industrial use—especially as polymer feedstocks.
References & Related Links
Related topics: Methods of Preparation of Hydrocarbons • Alkanes • Aromatic Hydrocarbons
External reading: LibreTexts Organic Chemistry
