Mechanism
Basics of Reaction Mechanism — CHEMASH
Reaction mechanism — the step-by-step sequence of elementary reactions converting reactants to products, showing intermediates and transition states.
Why learn reaction mechanisms?
They help chemists understand how and why a reaction occurs, predict products, and control conditions to improve yield or selectivity.
Key Terminology
- Reactants: Starting substances
- Products: Substances formed
- Intermediates: Temporary species formed during the reaction
- Transition state: High-energy configuration between reactant and product
- Reaction pathway: Series of steps from reactants to products
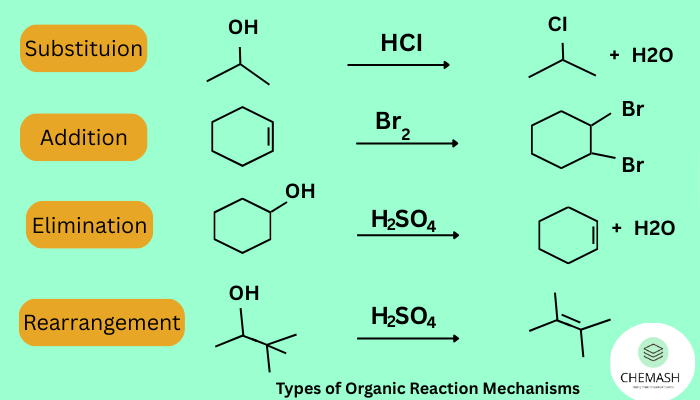
Types of Organic Reaction Mechanisms
- Nucleophilic Substitution (SN1 & SN2): A nucleophile replaces a leaving group
- Electrophilic Addition: Electrophile adds to a double/triple bond
- Elimination (E1 & E2): Removal of groups forming double/triple bonds
- Free Radical Reactions: Involves species with unpaired electrons
- Rearrangement Reactions: Atoms/groups shift positions in a molecule
Example: SN2 Reaction
In the reaction of CH3Br with OH-, the hydroxide ion attacks the carbon from the opposite side of the leaving group (Br-), forming CH3OH.
Key features: one-step reaction, no intermediates, backside attack leads to inversion of configuration.
Energy Profile Diagram
Energy diagrams show energetic changes during a reaction. Transition states are the highest points; intermediates appear as valleys between peaks. A multi-step reaction has multiple peaks (transition states) and valleys (intermediates). Reactants Transition State Products
Tip: Multi-step reactions may involve reactive intermediates like carbocations or carbanions.
Factors Affecting Reaction Mechanisms
- Nature of reactants: Size, charge, functional groups
- Solvent: Polar vs non-polar solvents influence ion stability
- Temperature: Affects rate and favored pathway
- Steric factors: Bulky groups can hinder attacks
- Electronic effects: Inductive and resonance effects influence reactivity
Common Intermediates
- Carbocations: Positively charged carbon species
- Carbanions: Negatively charged carbon species
- Free radicals: Neutral species with unpaired electrons
- Carbenes and nitrenes: Electron-deficient species
Applications of Reaction Mechanisms
- Designing drugs and pharmaceuticals
- Optimizing industrial synthesis
- Predicting and reducing side products
- Understanding biochemical pathways
Quiz: Basics of Reaction Mechanism
- What is a reaction mechanism?
- What are intermediates?
- Name one type of substitution reaction mechanism.
- What does an energy profile diagram show?
- What type of species is formed in free radical reactions?
- What is the main difference between SN1 and SN2?
- What factors influence a reaction mechanism?
- What is a transition state?
- Why is understanding mechanisms important?
- Give one real-world application of reaction mechanisms.
Answers:
- The detailed step-by-step process of how a reaction occurs
- Temporary species formed during the reaction pathway
- SN1 or SN2
- It shows the energy changes during a reaction step
- Free radicals (unpaired electrons)
- SN1 is two-step with a carbocation intermediate; SN2 is one-step with backside attack
- Nature of reactants, solvent, temperature, sterics, electronics
- A high-energy state between reactant and product
- To control reactions and predict products
- Drug synthesis, polymer production, biochemical studies
Published by CHEMASH • Last updated: September 6, 2025
Factors Affecting Reaction Mechanisms
- Nature of reactants: Size, charge, functional groups
- Solvent: Polar vs non-polar solvents influence ion stability
- Temperature: Affects rate and favored pathway
- Steric factors: Bulky groups can hinder attacks
- Electronic effects: Inductive and resonance effects influence reactivity
For deeper study, check resources like Khan Academy, ChemLibreTexts.
