Bonding in Coordination Compounds
Bonding in coordination compounds explains the interaction between a central metal ion and ligands. This bonding—through coordinate bonds, hybridization, and orbital interactions—determines geometry, color, stability, and reactivity of complexes.
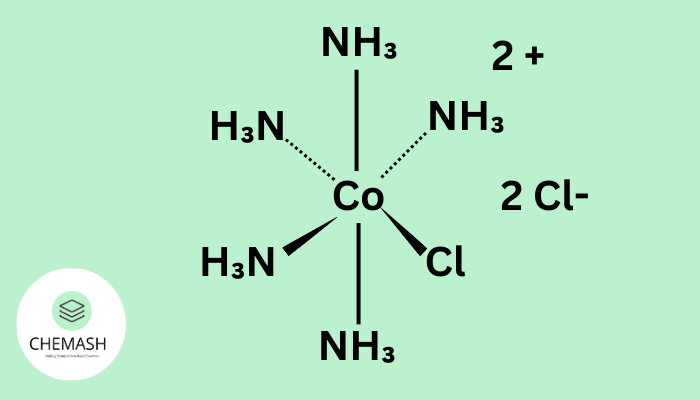
Table of Contents
- 1. Coordinate (Dative Covalent) Bond
- 2. Nature of Metal-Ligand Bonding
- 3. Valence Bond Theory (VBT)
- 4. Crystal Field Theory (CFT)
- 5. Molecular Orbital Theory (MOT)
- 6. Factors Affecting Bonding
- Quiz with Explanations
- MCQs with Answers
- FAQs
- External Links
1. Coordinate (Dative Covalent) Bond
The key bond in coordination compounds is the coordinate bond, where both electrons come from the ligand (Lewis base) and are accepted by the metal ion (Lewis acid).
Example: [Cu(NH3)4]2+ — ammonia donates lone pairs to copper.
2. Nature of Metal-Ligand Bonding
- Electrostatic Interaction: Attraction between positively charged metal and negatively charged ligand sites.
- Covalent Interaction: Electron pair donation into empty orbitals of the metal.
3. Valence Bond Theory (VBT)
Explains bonding through hybridization of metal orbitals.
- sp3: Tetrahedral
- dsp2: Square planar
- d2sp3: Octahedral
Example: [Ni(CN)4]2− → dsp2, square planar.
4. Crystal Field Theory (CFT)
Ligands are treated as point charges that split d-orbitals into energy levels.
- Octahedral field: Splitting into t2g and eg.
- Tetrahedral field: Splitting into e and t2.
Note: Strong field ligands → larger splitting (Δ), often low-spin complexes.
5. Molecular Orbital Theory (MOT)
Combines metal and ligand orbitals into molecular orbitals. Explains magnetic properties, bonding/antibonding orbitals, and spectra.
6. Factors Affecting Bonding
- Higher metal charge → stronger bonding
- Oxidation state increases bond strength
- Ligand donor/acceptor properties affect splitting
- Electronic configuration and metal size affect geometry
Quiz with Explanations
- Q: What bond is formed in coordination compounds?
A: Coordinate (dative covalent) bond. Ligand donates lone pair → metal accepts. - Q: How does VBT explain geometry?
A: Hybridization of orbitals (sp3, dsp2, d2sp3) → defines geometry. - Q: What causes d-orbital splitting in CFT?
A: Electrostatic repulsion from ligand fields. - Q: Example of dsp2 hybridization?
A: [Ni(CN)4]2−. - Q: How do strong field ligands affect Δ?
A: Increase Δ → often low-spin complexes.
(MCQs)
- The bond formed between a ligand and a metal ion is:
a) Ionic
b) Coordinate covalent ✅
c) Metallic
d) Hydrogen - Which hybridization corresponds to octahedral geometry?
a) sp3
b) dsp2
c) d2sp3 ✅
d) sp2 - Crystal field splitting occurs because ligands:
a) Donate electrons by sigma bonding
b) Act as point charges creating field ✅
c) Share electrons equally
d) None - Which complex is square planar?
a) [Fe(H2O)6]3+
b) [Ni(CN)4]2− ✅
c) [CoCl6]3−
d) [Zn(NH3)4]2+ - Strong field ligands cause:
a) Decreased splitting
b) Increased splitting ✅
c) No effect
d) Ionic bonds
FAQs
Q1: What is the main bond in coordination compounds?
Ans: Coordinate covalent bond formed by ligand electron donation.
Q2: How does CFT explain bonding?
Ans: By d-orbital splitting due to ligand electrostatic fields.
Q3: Which theory explains spectra better?
Ans: Molecular Orbital Theory (MOT).
Q4: Example of dsp² hybridization?
Ans: [Ni(CN)4]2−, square planar.
Q5: Effect of strong field ligands?
Ans: Larger splitting (Δ), low-spin complexes.
Valence Bond Theory – Britannica
