Catalysis
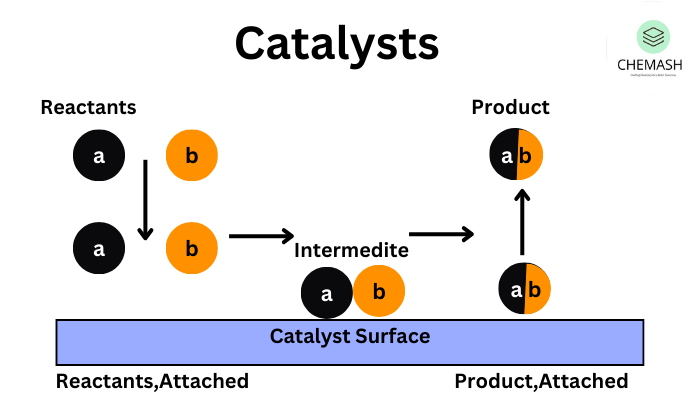
Catalysis is a process in which the rate of a chemical reaction is increased by the presence of a substance called a catalyst. The catalyst participates in the reaction but remains chemically unchanged at the end. This is a fundamental concept in surface chemistry and plays a vital role in both laboratory and industrial reactions.
Types of Catalysis
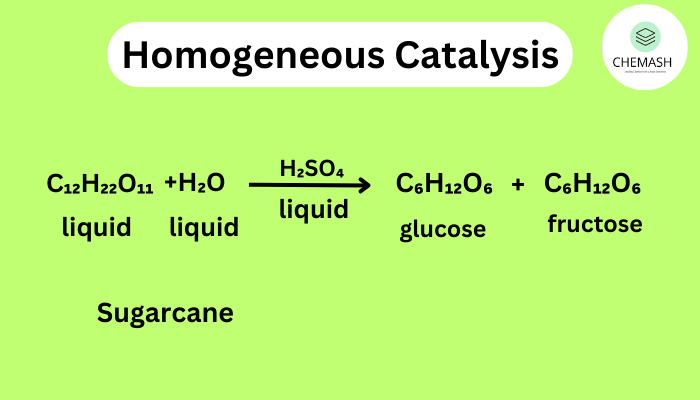
- Homogeneous Catalysis: Catalyst and reactants are in the same phase (e.g., liquid-liquid).
- Heterogeneous Catalysis: Catalyst is in a different phase than reactants (e.g., solid catalyst with gas reactants).
- Autocatalysis: One of the products acts as a catalyst for the same reaction.
- Enzyme Catalysis: Biological catalysts that speed up biochemical reactions.
Characteristics of Catalysts
- Remain unchanged in mass and composition after the reaction.
- Do not initiate the reaction; only speed it up.
- Effective in small amounts.
- Highly specific — one catalyst works for one type of reaction.
- Often lower the activation energy.
Mechanism of Catalysis (Heterogeneous)
- Adsorption: Reactant molecules are adsorbed onto the catalyst’s surface.
- Activation: Bonds in reactants weaken due to surface interaction.
- Reaction: Chemical change occurs at the active site.
- Desorption: Products leave the surface, and the catalyst is free for reuse.
Industrial Applications of Catalysis
- Haber’s process: Fe catalyst for ammonia production.
- Contact process: V2O5 for sulfuric acid manufacture.
- Hydrogenation of oils: Ni catalyst to convert vegetable oils to ghee/margarine.
- Catalytic converters: Platinum and palladium to convert toxic gases to harmless products in vehicle exhaust.
Enzyme Catalysis
Enzymes are biological catalysts working under mild conditions (pH, temperature) and are highly specific.
- Amylase: Breaks starch into sugar.
- Protease: Breaks proteins into amino acids.
- DNA polymerase: Catalyzes DNA replication.
Importance of Catalysis
- Speeds up chemical reactions, increasing productivity.
- Enables eco-friendly processes by lowering energy needs.
- Essential for sustainable development and green chemistry.
- Vital in pharmaceuticals, food processing, and environmental cleanup.
Conclusion:
Catalysis is central to modern chemistry, with vast industrial, biological, and environmental applications. Understanding catalyst behavior and surface interactions leads to faster, cleaner, and more efficient chemical processes.
Quick MCQ Quiz
- Which catalyst is used in Haber’s process?
a) Platinum
b) Iron ✅
c) Nickel
d) Copper - Enzyme catalysis is a type of:
a) Homogeneous catalysis ✅
b) Heterogeneous catalysis
c) Autocatalysis
d) None - What is the main role of a catalyst?
a) Increase activation energy
b) Decrease activation energy ✅
c) Start the reaction
d) Increase temperature - Which catalyst is used in hydrogenation of oils?
a) Ni ✅
b) Pt
c) Fe
d) V2O5 - Catalytic converters use:
a) Iron
b) Nickel
c) Platinum & Palladium ✅
d) Cobalt
True / False
- Catalysts are consumed in the reaction. ❌ (False – They remain unchanged)
- Heterogeneous catalysis involves reactants and catalysts in the same phase. ❌ (False – Different phases)
- Enzymes are biological catalysts. ✅ (True)
