Charles’s Law – Definition, Formula, Graph, Examples & Applications
Charles’s Law is one of the fundamental gas laws in physics and chemistry. It explains the relationship between the volume and temperature of a gas when the pressure remains constant.
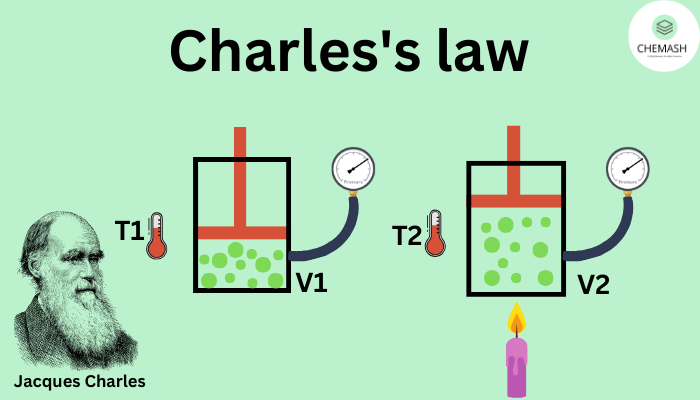
What is Charles’s Law?
Charles’s Law states that:
At constant pressure, the volume of a fixed mass of gas is directly proportional to its absolute temperature.
This means that as the temperature of a gas increases, its volume also increases, and when the temperature decreases, the volume decreases—provided the pressure does not change.
Mathematical Expression of Charles’s Law
The law can be written as:
V ∝ T
Or in equation form:
V / T = Constant
For two different states of the gas:
V₁ / T₁ = V₂ / T₂
- V = Volume of gas
- T = Absolute temperature (in Kelvin)
- Pressure = Constant
⚠️ Important: Temperature must always be measured in Kelvin (K), not Celsius.
Charles’s Law Graph
A graph between Volume (V) and Temperature (T) at constant pressure is a straight line passing through the origin when temperature is measured in Kelvin.
- Slope of the graph = V/T (constant)
- Linear increase shows direct proportionality
Why Absolute Temperature is Used?
Absolute temperature (Kelvin) starts from absolute zero (0 K), where molecular motion theoretically stops. Using Celsius would give incorrect results because it does not start from zero molecular energy.
Real-Life Applications of Charles’s Law
- Hot air balloons: Heating air increases volume, making the balloon rise.
- Car tyres: Tyres expand in hot weather due to increased temperature.
- Inflated balloons: Shrink in cold and expand in heat.
- Gas storage tanks: Designed keeping temperature expansion in mind.
Numerical Example
A gas has a volume of 2.0 m³ at 300 K. What will be its volume at 450 K at constant pressure?
Solution:
Using Charles’s Law:
V₁ / T₁ = V₂ / T₂
2.0 / 300 = V₂ / 450
V₂ = (2.0 × 450) / 300 = 3.0 m³
Limitations of Charles’s Law
- Applies only to ideal gases
- Fails at very high pressure
- Fails at very low temperature where gases liquefy
Difference Between Charles’s Law and Boyle’s Law
| Charless Law | Boyle’s Law |
|---|---|
| Volume ∝ Temperature | Volume ∝ 1/Pressure |
| Pressure constant | Temperature constant |
MCQs
- According to Charless Law, volume is directly proportional to:
A. Pressure
B. Temperature
C. Mass
D. Density - Which temperature scale must be used in Charless Law?
A. Celsius
B. Fahrenheit
C. Kelvin
D. Rankine
Answers with Explanation
Q1: B – Volume is directly proportional to temperature.
Q2: C – Kelvin is used because it is an absolute temperature scale.
Frequently Asked Questions (FAQs)
What remains constant in Charless Law?
Pressure and amount of gas remain constant.
What happens to volume at absolute zero?
According to Charless Law, volume theoretically becomes zero at 0 K.
Is Charless Law valid for real gases?
It is approximately valid at low pressure and high temperature.
