Acids and bases are fundamental chemical compounds that play crucial roles in laboratories, industries, and biological systems. The classification of acids and bases is done based on strength, concentration, acidity/basicity, source, and scientific theories like Arrhenius theory, Bronsted-Lowry, and Lewis theory.
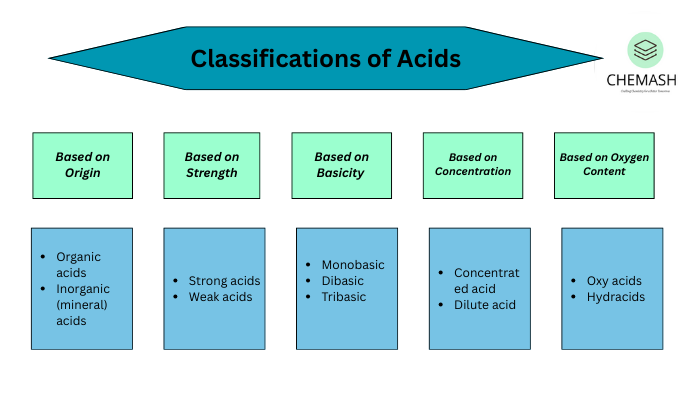
1. Classification of Acids and Bases Based on Strength
- Strong Acids: Completely dissociate in water. Examples: HCl, HNO₃, H₂SO₄.
- Weak Acids: Partially dissociate in water. Examples: CH₃COOH, H₂CO₃.
- Strong Bases: Completely dissociate to produce OH⁻ ions. Examples: NaOH, KOH.
- Weak Bases: Partially dissociate in water. Examples: NH₄OH, Ca(OH)₂.
2. Classification of Acids and Bases Based on Concentration
- Concentrated Acids/Bases: Contain a large amount of acid/base in a given volume of water.
- Dilute Acids/Bases: Contain a small amount of acid/base in a large volume of water.
3. Classification of Acids and Bases Based on Basicity or Acidity
- Monobasic Acids: Yield one H⁺ ion per molecule. Example: HCl, HNO₃.
- Dibasic Acids: Yield two H⁺ ions per molecule. Example: H₂SO₄.
- Tribasic Acids: Yield three H⁺ ions per molecule. Example: H₃PO₄.
- Monacidic Bases: Contain one OH⁻ ion. Example: NaOH.
- Diacidic Bases: Contain two OH⁻ ions. Example: Ca(OH)₂.
4. Classification of Acids and Bases Based on Source
- Organic Acids: Derived from living organisms. Example: Citric acid, Acetic acid.
- Inorganic (Mineral) Acids: Derived from minerals. Example: HCl, H₂SO₄.
- Organic Bases: Contain nitrogen and carbon; examples include amines.
- Inorganic Bases: Commonly metal hydroxides like NaOH, Ca(OH)₂.
5. Arrhenius Classification
According to the Arrhenius theory:
- Acid: Increases H⁺ concentration in aqueous solution.
- Base: Increases OH⁻ concentration in aqueous solution.
6. Bronsted-Lowry Classification of Acids and Bases
According to the Bronsted-Lowry theory:
- Acid: Proton (H⁺) donor.
- Base: Proton (H⁺) acceptor.
7. Lewis Classification
According to the Lewis theory:
- Acid: Electron pair acceptor.
- Base: Electron pair donor.
Conclusion
The classification of acids and bases provides a scientific framework to understand their reactivity and applications in industry, medicine, agriculture, and daily life.
Quiz
Q1: Which of the following is a weak acid?
A) HCl
B) HNO₃
C) CH₃COOH
D) H₂SO₄
Answer: C) CH₃COOH
Explanation: Acetic acid (CH₃COOH) only partially dissociates in water.
Q2: Which acid is tribasic?
A) HCl
B) HNO₃
C) H₂SO₄
D) H₃PO₄
Answer: D) H₃PO₄
Explanation: H₃PO₄ releases three protons.
Q3: According to Bronsted-Lowry theory, a base is:
A) Proton donor
B) Proton acceptor
C) Electron pair acceptor
D) Hydroxide producer
Answer: B) Proton acceptor
Explanation: Bases accept H⁺.
Q4: Which is a Lewis base?
A) HCl
B) NH₃
C) HNO₃
D) H₂SO₄
Answer: B) NH₃
Explanation: NH₃ donates a lone pair of electrons.
FAQ
Q1: What is the difference between strong and weak acids?
Strong acids fully dissociate in water, while weak acids only partially ionize.
Q2: Which theory defines acids as electron pair acceptors?
The Lewis theory defines acids as electron pair acceptors.
Q3: Give one example of an organic acid.
Acetic acid (CH₃COOH) is a common organic acid.
