Chemical Bonding – Coordinate Bond (Dative Bond)
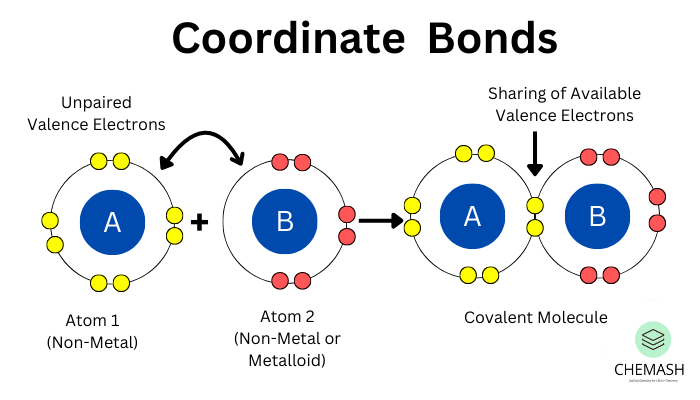
A coordinate bond, also known as a dative covalent bond, is a type of covalent bond in which both electrons in the shared pair come from the same atom. It is commonly observed when a lone pair from one atom is donated to an electron-deficient species.
Formation
- Donor: Atom with a lone pair of electrons (N, O, etc.).
- Acceptor: Electron-deficient atom or ion (e.g., H⁺, metal cations).
Representation: Denoted by an arrow (→) from donor to acceptor atom.
Examples
- Ammonium ion (NH4+):
NH3 + H+ → NH4+ - Hydronium ion (H3O+): Water donates a lone pair to H+.
- Carbon monoxide (CO): O donates lone pair to C forming a triple bond (one coordinate).
- Complex ions: [Cu(NH3)4]2+, where NH3 donates lone pairs.
Properties
- Behaves like a covalent bond after formation.
- Common in coordination compounds.
- Observed in Lewis acid-base reactions.
- Important in transition metal chemistry and polyatomic ions.
Quiz: Test Your Knowledge
- What makes a coordinate bond different from a covalent bond?
- Which ion is formed when NH3 donates a lone pair to H+?
- What symbol represents a coordinate bond?
- Give two examples of coordinate bonded compounds.
- Explain bonding in H3O+.
Answers
- Both electrons in a coordinate bonds come from the donor atom.
- Ammonium ion (NH4+).
- Arrow (→) from donor to acceptor.
- NH4+, CO, H3O+, [Cu(NH3)4]2+.
- Oxygen donates a lone pair to H⁺ → H3O+.
FAQs
Q1: Is a coordinate bonds weaker than a covalent bond?
Ans: No, once formed, a coordinate bond is indistinguishable from a covalent bond in strength.
Q2: How do you identify a coordinate bond?
Ans: Look for lone pairs donated from one atom to an electron-deficient atom (represented by →).
Q3: Where are coordinate bonds commonly found?
Ans: In complex ions, polyatomic ions, and Lewis acid-base reactions.
Read also: Bond Parameters | Inorganic Chemistry
