Introduction to Coordination Compounds
The Introduction to coordination compounds explains how a central metal atom or ion binds to ligands (molecules or ions that donate electron pairs) to form coordinate covalent bonds; this overview uses IUPAC-friendly terminology.
Table of Contents
- Key Features
- Examples
- Importance & Applications
- Quiz (interactive)
- MCQs (with answers)
- FAQ
- References & Links
Key Features of Coordination Compounds
- Central Metal Ion: Usually a transition metal which acts as a Lewis acid (electron-pair acceptor).
- Ligands: Atoms, ions, or molecules with lone pairs (Lewis bases) that donate to the metal.
- Coordinate Bond: Covalent bond where both electrons originate from the ligand.
- Coordination Number: Number of ligand donor atoms attached to the metal.
- Complex Ion: A coordination entity carrying a net charge (cationic or anionic).
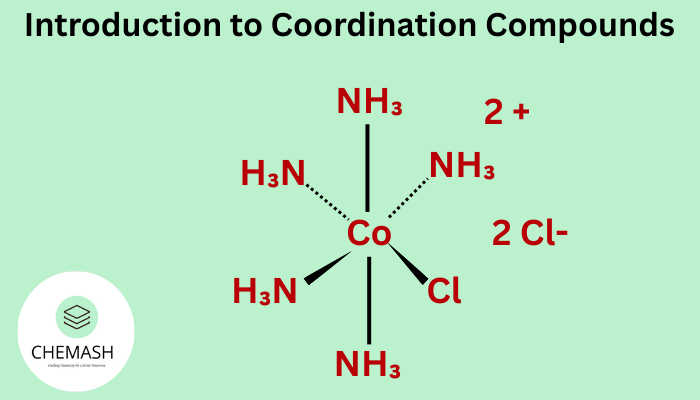
Examples
- [Cu(NH3)4]2+ – Tetraamminecopper(II) ion
- [Fe(CN)6]3− – Hexacyanoferrate(III) ion
- [Co(H2O)6]3+ – Hexaaquacobalt(III) ion
Importance & Applications
Coordination compounds are vital across chemistry and industry:
- Catalysis — e.g., Wilkinson’s catalyst for hydrogenation.
- Medicine — e.g., cisplatin (a platinum coordination compound) used in chemotherapy.
- Biological systems — e.g., hemoglobin contains an iron coordination complex (heme).
- Industrial uses — dyes, pigments, analytical reagents and extraction processes.
Quiz: Test Your Knowledge (interactive)
Q1: What is a ligand in coordination chemistry?
Answer: An atom, ion or molecule that donates an electron pair to the central metal ion.
Explanation: Ligands act as Lewis bases; they supply both electrons for the coordinate bond.
Q2: Define coordination number.
Answer: The number of ligand donor atoms directly bonded to the central metal.
Explanation: A bidentate ligand contributes two donor atoms to the coordination number.
Q3: Name a coordination compound in biological systems.
Answer: Hemoglobin (iron-centered heme complex).
Explanation: The heme group coordinates Fe2+ with nitrogen donors from the porphyrin ring.
Multiple Choice Questions (MCQs)
- Which of the following best describes a ligand?
a) Electron pair acceptor
b) Electron pair donor
c) Free radical
d) Metal ion
Explanation: Ligands donate an electron pair to the metal, so they are electron-pair donors (Lewis bases). - Coordination number refers to:
a) Number of ligand donor atoms bonded to the metal
b) Number of electrons in metal
c) Charge on the complex
d) Number of atoms in ligand
Explanation: Coordination number counts donor atoms attached directly to the metal centre. - What kind of bond is formed in coordination compounds?
a) Ionic bond
b) Coordinate covalent bond
c) Metallic bond
d) Hydrogen bond
Explanation: The shared pair of electrons both come from the ligand — a coordinate covalent (dative) bond. - Which metal ion is commonly found as the central atom in coordination compounds?
a) Alkali metals
b) Transition metals
c) Noble gases
d) Halogens
Explanation: Transition metals have variable oxidation states and available d-orbitals suited to coordination. - Hemoglobin contains which central metal in its coordination complex?
a) Copper
b) Zinc
c) Iron
d) Magnesium
Explanation: Hemoglobin’s heme group contains iron (Fe), which coordinates to the porphyrin ring and oxygen.
MCQ Answers
- b) Electron pair donor
- a) Number of ligand donor atoms bonded to the metal
- b) Coordinate covalent bond
- b) Transition metals
- c) Iron
Frequently Asked Questions (FAQ)
Q: Why are transition metals common central atoms?
A: Transition metals have variable oxidation states and available d-orbitals, enabling diverse ligand binding and complex geometries.
Q: What is the difference between a coordinate covalent bond and a regular covalent bond?
A: In a coordinate covalent bond both bonding electrons originate from the same atom (the ligand); in typical covalent bonds electrons are shared with contributions from both atoms.
Q: Where can I study official nomenclature rules?
A: See the IUPAC recommendations and the IUPAC Nomenclature of Inorganic Chemistry for authoritative rules. (IUPAC)
- Official IUPAC guidance — IUPAC (external)
- CHEMASH: Nomenclature of Coordination Compounds (internal)
© 2025 CHEMASH. All Rights Reserved.
Trusted Chemistry Learning Platform | NEET • JEE • CUET
