Nomenclature of Coordination Compounds
The nomenclature of coordination compounds is a systematic method used in coordination chemistry to name these compounds according to their composition and structure. Following the standardized rules set by the International Union of Pure and Applied Chemistry (IUPAC), this nomenclature ensures clarity and consistency in identifying coordination complexes.
Table of Contents
- Basic Rules for Naming
- Ligand names & prefixes
- Metal name & oxidation state
- Examples
- Quiz (interactive)
- MCQs (with explanations)
- FAQ
- References & links
Basic Rules for Naming Coordination Compounds
- Name the ligands first: List ligands before the central metal; order ligands alphabetically (ignore numeric prefixes).
- Ligand names:
- Neutral ligands: keep usual names — NH3 → ammine, H2O → aqua, CO → carbonyl.
- Anionic ligands: use suffix -o — Cl– → chloro, OH– → hydroxo, CN– → cyano.
- Polydentate ligands keep common abbreviations: ethylenediamine → en.
- Numbers of ligands: mono-, di-, tri-, tetra-, penta-, hexa-. For complex names use bis-, tris-, tetrakis- when needed.
- Name the central metal: If the complex is cationic or neutral, metal name stays (e.g., cobalt). For anionic complexes use -ate (e.g., ferrate).
- Oxidation state: Give oxidation state in Roman numerals immediately after the metal name, e.g.,
cobalt(III). - Ionic compounds: Name the cation first, then the anion (as usual).
Ligand names & numeric prefixes
- mono-, di-, tri- etc. are used when ligand names are simple (ignore prefix order when alphabetising).
- Use bis-, tris-, tetrakis- for ligands which themselves contain prefixes or punctuation (e.g., bis(ethylenediamine)).
Metal names & oxidation states
When the complex is an anion, add -ate to the metal and sometimes use Latin stem for older metal names (e.g., iron → ferrate). Always append the oxidation state in Roman numerals.
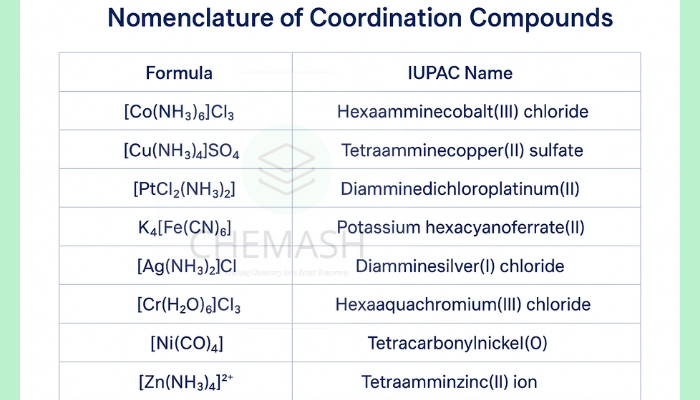
Examples (with IUPAC-style names)
- [Co(NH3)6]Cl3 → Hexaamminecobalt(III) chloride
- [Fe(CN)6]4− → Hexacyanoferrate(II) ion
- [Cu(H2O)4Cl2] → Tetraaquadichlorocuprate(II)
- [PtCl2(NH3)2] → Diamminedichloroplatinum(II)
- [Cr(en)2(H2O)2]Cl3 → Diethylenediaminediaquachromium(III) chloride
Tip: Ignore numeric prefixes when alphabetizing ligands but count them for naming (e.g., ammine before chloro if alphabetically appropriate).
Quiz: Test Your Understanding (interactive)
Q1: Name the complex ion: [Ni(CO)4]
Answer:Tetracarbonylnickel(0)
Explanation: CO is named carbonyl; four carbonyl ligands → tetracarbonyl. Nickel here has oxidation state 0.Q2: What is the correct name for [Cr(H2O)6]Cl3?
Answer:Hexaaquachromium(III) chloride
Explanation: Six aqua ligands → hexaaqua; chromium oxidation state +3 → (III); chloride is the counterion.Q3: Name the complex: [Cu(en)2Cl2]
Answer:Bis(ethylenediamine)dichlorocopper(II)
Explanation: ‘en’ (ethylenediamine) is a bidentate ligand → two such ligands → bis(en); two chloro ligands → dichloro; copper oxidation state +2.Q4: What prefix is used if a complex has two ethylenediamine ligands?
Answer:Bis-
Explanation: Because ‘ethylenediamine’ is a polydentate ligand and contains a hyphen/complex name, use ‘bis-‘.
Multiple Choice Questions (MCQs) — with explanations
- Which suffix is used for the metal in an anionic complex?
a) -ium
b) -ate
c) -ide
d) -ous
Explanation: Anionic complexes use the suffix-ate(e.g., ferrate). - What is the correct name for
[Co(NH3)5Cl]2+?
a) Pentaamminochlorocobalt(II)
b) Pentaamminechlorocobalt(III)
c) Pentaminechlorocobalt(III)
d) Pentamminochlorocobalt(III)
Explanation: With 5 NH3 (neutral) + 1 Cl− (anionic) and overall +2 charge, cobalt must be in +3 oxidation state → cobalt(III). Use ‘ammine’ spelling for NH3 ligands. - Which ligand name corresponds to H2O?
a) NH3
b) H2O (aqua)
c) CO
d) CN−
Explanation: H2O is named ‘aqua’ in coordination nomenclature. - What prefix is used for three ligands of the same kind (when name is simple)?
a) Tri-
b) Tetra-
c) Tri-
d) Bis-
Explanation: Usetri-for three identical simple ligands. Use ‘tris-‘ primarily for complex ligand-names. - What is the oxidation state of the metal in
[Fe(CN)6]4−?
a) +2
b) +2
c) +4
d) +6
Explanation: Each CN− is −1, total ligand charge = −6. Complex overall is −4, so Fe must be +2 to make net −4.
(FAQ)
Q: Why do some metal names change (e.g., ferrate)?
A: When a coordination complex is an anion, tradition and IUPAC practice turn the metal name into an ‘-ate’ form (often using Latin stem): iron → ferrate, copper → cuprate. This signals anionic complex. Q: Do you alphabetise ‘bis-‘ and ‘tri-‘ when ordering ligands?
A: No — alphabetise by ligand name ignoring numeric prefixes (e.g., ‘ammine’ before ‘chloro’). Use ‘bis-‘, ‘tris-‘ only to count complex ligand groups, not for alphabetising. Q: Where can I read the official IUPAC rules?
A: The official IUPAC recommendations and IUPAC Nomenclature of Inorganic Chemistry documents contain comprehensive rules. See IUPAC’s website for the latest recommendations. (IUPAC)

I’ve been exploring for a little bit for any high-quality articles or weblog posts on this kind of space . Exploring in Yahoo I ultimately stumbled upon this website. Studying this information So i’m glad to convey that I’ve a very just right uncanny feeling I found out exactly what I needed. I most indubitably will make certain to do not forget this site and provides it a look on a constant basis.
This was really helpful, I had some confusions in naming coordination compounds. But after going through this, I can say I’ve learnt a lot.