Stability of Coordination Compounds
The stability of coordination compounds indicates how strongly a metal ion holds its ligands and resists ligand substitution or decomposition; stability is vital in catalysis, biology, and industry.
Table of Contents
- Types of Stability
- Factors Affecting Stability
- Formation Constant (Kf)
- Chelate Effect
- Quiz
- MCQs
- FAQ
- References & Links
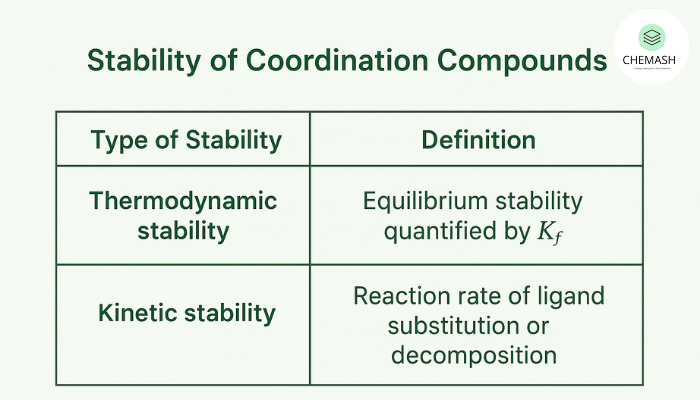
Types of Stability
- Thermodynamic stability: Equilibrium stability quantified by the formation (stability) constant, Kf.
- Kinetic stability: Reaction rate of ligand substitution or decomposition — complexes can be thermodynamically stable but kinetically labile (or vice versa).
Factors Affecting Stability
- Nature of metal ion: Higher charge and smaller ionic radius generally increase stability.
- Nature of ligands: Strong-field ligands (e.g., CN–) form more stable complexes than weak-field ligands (e.g., H2O).
- Chelate effect: Polydentate ligands increase stability by entropy gain and ring formation.
- Oxidation state: Higher oxidation states often give stronger attraction and more stable complexes.
- Geometric & electronic factors: Crystal Field Stabilisation Energy (CFSE) and preferred geometries (octahedral, square planar) influence stability.
- Solvent & competing ligands: Solvent polarity and presence of other ligands can shift equilibria.
Formation Constant (Stability Constant)
The equilibrium for complex formation:
Mn+ + xL ⇌ [MLx]n+
Kf = [complex] / ([Mn+][L]x)
A larger Kf value means the complex is thermodynamically more stable at equilibrium.
Chelate Effect
Chelating ligands (bidentate/polydentate) form more stable complexes than equivalent monodentate ligands. Reasons:
- Entropy gain: formation displaces more particles into solution.
- Ring formation: multidentate binding forms stable rings that resist dissociation.
Example: ethylenediamine (en) typically forms more stable complexes than NH3 with the same metal.
Quiz: Test Your Knowledge
Q1: What is the difference between thermodynamic and kinetic stability?
Answer: Thermodynamic stability is about equilibrium favorability (Kf); kinetic stability is about how rapidly a complex undergoes substitution or decomposition.
Explanation: A complex can be very stable thermodynamically (high Kf) but still exchange ligands quickly (kinetically labile)
.Q2: How does the charge on the metal ion affect stability?
Answer: Higher positive charge increases electrostatic attraction to ligands, usually increasing stability.
Explanation: More highly charged metals bind ligands more strongly, all else equal.
Q3: Explain the chelate effect.
Answer: Polydentate ligands form rings and increase entropy on formation, producing more stable complexes than equivalent monodentate ligands.
Explanation: Example — en vs NH3 for the same metal.
Q4: What does a high formation constant (Kf) signify?
Answer: The complex is strongly favored at equilibrium — thermodynamically stable.
Explanation: Higher Kf → greater complex concentration relative to free metal and ligand.
Q5: Give an example of a polydentate ligand and its advantage.
Answer: Ethylenediamine (en) — advantage: forms 5-membered chelate rings and increases complex stability.
Explanation: Chelation improves both thermodynamic and often kinetic stability.
Multiple Choice Questions — with explanations
- Thermodynamic stability refers to:
a) The equilibrium favorability of complex formation
b) The speed of ligand substitution
c) The magnetic property of the complex
d) The color of the complex
Explanation: Thermodynamic stability is quantified by Kf. - Which factor increases the stability of coordination compounds?
a) Larger ionic radius of metal
b) Lower charge on metal ion
c) Chelate effect
d) Monodentate ligands only
Explanation: Chelation typically stabilizes complexes through entropy and ring formation. - A high formation constant (Kf) indicates:
a) Low stability
b) No reaction
c) High stability
d) Rapid decomposition
Explanation: Higher Kf means equilibrium lies toward the complex. - Which of the following is a polydentate ligand?
a) NH3
b) Ethylenediamine (en)
c) Cl−
d) H2O
Explanation: en is bidentate (two donor atoms) and can chelate. - Kinetic stability relates to:
a) How energetically favorable a complex is
b) How fast it reacts or decomposes
c) The charge on the metal ion
d) The geometry of the complex
Explanation: Kinetic measures reaction rates for ligand exchange or decomposition.
MCQ Answers
- a) The equilibrium favorability of complex formation
- c) Chelate effect
- c) High stability
- b) Ethylenediamine (en)
- b) How fast it reacts or decomposes
Frequently Asked Questions
Q: Can a complex be thermodynamically stable but kinetically labile?
A: Yes — an example is some octahedral d⁶ complexes that exchange ligands rapidly despite high Kf under certain conditions.
Q: How do solvents affect stability?
A: Polar solvents can stabilize charged species and compete with ligands; coordinating solvents (e.g., water, DMSO) may displace weak ligands and change equilibria.
Q: Where can I read more about formation constants and experimental methods?
A: See textbooks on coordination chemistry and analytical methods (potentiometry, spectrophotometry). Authoritative guidance is available on IUPAC (external). Also useful internal resources: Ligands & Chelation.
