Coordination Number
Table of Contents
- Definition
- Importance of Coordination Number
- Common Coordination Numbers
- Factors Affecting Coordination Number
- Coordination Number and Geometry
- Examples
- Quiz
- MCQs
- FAQs
Definition
The coordination number of a coordination compound is the number of ligand donor atoms directly bonded to the central metal ion in a complex. It represents the total number of points of attachment of ligands to the metal.
Importance of Coordination Numbers
The coordination numbers determines the geometry, stability, magnetic properties, and reactivity of the compound. Different numbers lead to different molecular shapes and influence chemical behavior.
Common Coordination Numbers
- 2: Linear geometry (e.g., [Ag(NH3)2]+)
- 4: Tetrahedral ([NiCl4]2−) or square planar ([Pt(NH3)2Cl2])
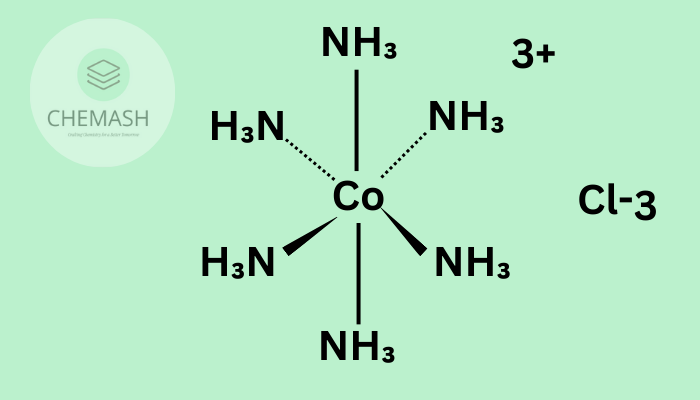
- 6: Octahedral ([Fe(H2O)6]3+)
- Others: 3, 5, 7, 8, 12 (less common)
Factors Affecting Coordination Numbers
- Size of Metal Ion: Larger ions accommodate more ligands.
- Size of Ligands: Bulky ligands reduce coordination numbers.
- Electronic Configuration: Influences metal’s ability to accept electron pairs.
- Oxidation State: Higher states often increase coordination numbers.
- Nature of Ligands: Chelating ligands can increase effective CN.
Coordination Number and Geometry
| Coordination Number | Geometry |
|---|---|
| 2 | Linear |
| 3 | Trigonal planar / Trigonal pyramidal |
| 4 | Tetrahedral / Square planar |
| 5 | Trigonal bipyramidal / Square pyramidal |
| 6 | Octahedral |
Examples
- [Cu(NH3)4]2+: CN = 4, square planar
- [Co(H2O)6]3+: CN = 6, octahedral
- [Ag(NH3)2]+: CN = 2, linear
Quiz: Test Your Knowledge
- What is the coordination number in a complex?
- Which coordination numbers is most common in transition metal complexes?
- How does the size of the central ion affect coordination number?
- Name two geometries for CN = 4.
- Why do bulky ligands reduce coordination number?
Answers
- Number of ligand donor atoms bonded to the central ion.
- 6 (octahedral).
- Larger ions allow more ligands.
- Tetrahedral, square planar.
- They occupy more space, limiting ligand binding.
(MCQs)
- Coordination numbers of [Fe(CN)6]4−?
a) 4
b) 6
c) 2
d) 8 - Which factor does NOT affect CN?
a) Size of ion
b) Nature of ligands
c) Color of complex
d) Oxidation state - CN = 2 usually gives:
a) Linear
b) Octahedral
c) Tetrahedral
d) Square planar - Which geometry is NOT possible for CN = 4?
a) Tetrahedral
b) Square planar
c) Octahedral
d) None - Bulky ligands generally:
a) Increase CN
b) Decrease CN
c) No effect
d) Change oxidation state
MCQ Explanations
- [Fe(CN)6]4− has 6 ligands, so CN = 6.
- Color depends on d-d transitions, not CN.
- Two ligands align linearly around the metal.
- CN = 4 cannot form octahedral (requires 6 ligands).
- Bulky ligands crowd the metal, reducing CN.
FAQs
What is coordination numbers in chemistry?
It is the number of ligand donor atoms bonded to the central metal ion.
Which coordination numbers is most common?
6, generally giving an octahedral geometry.
What factors affect coordination numbers?
Size of metal ion, size and type of ligands, oxidation state, and electronic configuration.
Wikipedia: Coordination Number.
