Chemical Bonding – Covalent Bond
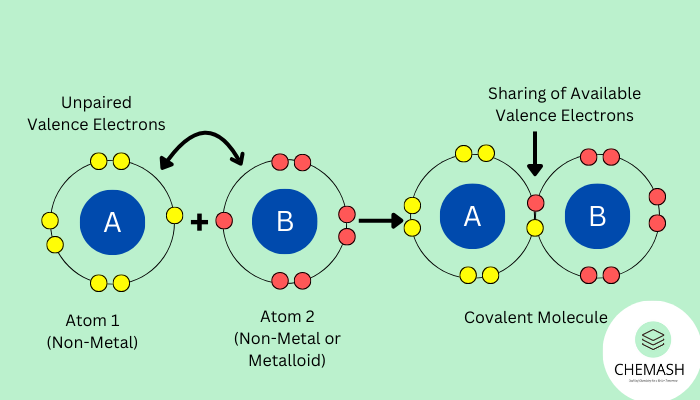
A covalent bond is a type of chemical bond formed when two atoms share one or more pairs of electrons. It usually occurs between nonmetals with similar electronegativities. Consequently, covalent bonding plays a vital role in the formation of molecules and molecular compounds. Unlike the ionic bond, which depends on electron transfer, covalent bonds rely on sharing.
Characteristics of Covalent Bonding
- It generally occurs between two non-metallic atoms.
- Instead of transfer, the atoms share electrons.
- As a result, molecules (discrete entities) are formed.
- The bonds may be single, double, or triple depending on the number of shared electron pairs.
- Through sharing, atoms achieve noble gas configuration (octet or duplet rule).
Types of Covalent Bonds
- Single Covalent Bond: One pair of electrons is shared (e.g., H2, Cl2, CH4).
- Double Covalent Bond: Two pairs of electrons are shared (e.g., O2, CO2).
- Triple Covalent Bond: Three pairs of electrons are shared (e.g., N2, C2H2).
Examples of Covalent Molecules
- Hydrogen (H2): Each hydrogen atom shares one electron, therefore forming a single bond.
- Oxygen (O2): Each oxygen shares two electrons, thus forming a double bond.
- Nitrogen (N2): Each nitrogen shares three electrons, consequently creating a very stable triple bond.
- Methane (CH4): Carbon shares electrons with four hydrogen atoms via single covalent bonds.
Bond Properties
- Bond Length: It refers to the distance between the nuclei of bonded atoms, and it decreases with increasing bond order.
- Bond Energy: This is the energy required to break the bond. Therefore, triple bonds are stronger than double, and double are stronger than single.
- Bond Angle: It is explained by VSEPR theory, which helps predict molecular geometry.
Polar and Nonpolar Covalent Bonds
Covalent bonds can be classified as:
- Nonpolar: Equal sharing of electrons occurs between identical atoms or atoms with similar electronegativities (e.g., H2, Cl2).
- Polar: Unequal sharing of electrons happens when there is a difference in electronegativity. As a result, partial positive and negative charges develop (e.g., HCl, H2O).
Lewis Dot Structures
Chemists often represent covalent bonding using Lewis structures, where shared pairs of electrons are drawn as lines or dots between atoms, and lone pairs are also displayed. (Learn more at Khan Academy)
Example: For H2O:
H : O : H → The oxygen atom shares two electrons (one each) with two hydrogen atoms, and it also keeps two lone pairs.
Importance of Covalent Bonding
- It forms the foundation of organic and molecular chemistry.
- Moreover, it provides the basis for complex biomolecules like DNA and proteins.
- It also explains molecular shapes, reactivity, and polarity.
- In addition, covalent bonding is widely applied in drug design, material science, and nanotechnology.
- Finally, it connects with metallic bonding and ionic bonding, making it a crucial part of bonding studies.
Quiz: Test Your Understanding
- Define a covalent bond with an example.
- Differentiate between single, double, and triple covalent bonds.
- What is the difference between polar and nonpolar covalent bonds?
- Why do covalent compounds usually have low melting points?
- Draw the Lewis structure for NH3 (Ammonia).
Answers
- A covalent bond forms when atoms share electrons. Example: H2.
- Single = 1 shared pair, Double = 2 shared pairs, Triple = 3 shared pairs of electrons.
- Polar bonds involve unequal sharing (e.g., HCl), whereas nonpolar bonds involve equal sharing (e.g., Cl2).
- Covalent compounds usually have low melting points because molecules are held together by weak van der Waals forces, rather than strong ionic attractions.
- NH3: Nitrogen is in the center with three single bonds to hydrogen and one lone pair.
