Crystal Lattices and Unit Cell
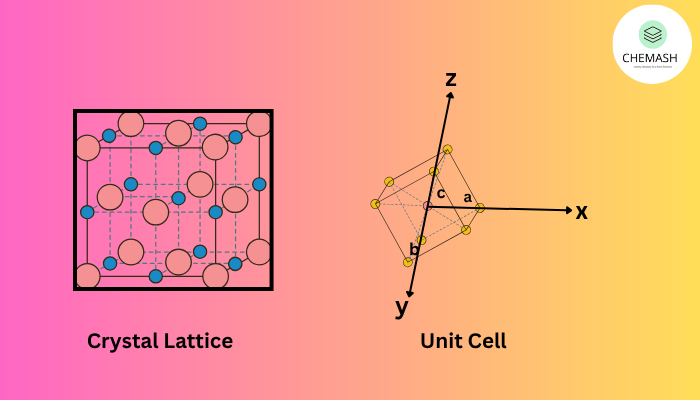
In solid state chemistry, the arrangement of particles in a crystalline solid is highly ordered and repetitive. This regular 3D arrangement is called a crystal lattice, and the smallest repeating unit that defines the lattice is called a unit cell.
What is a Crystal Lattice?
A crystal lattice is a geometric pattern formed by atoms, ions, or molecules in crystalline materials. Each point (called a lattice point) represents the position of a particle. The lattice extends in 3D space.
Key Features of a Crystal Lattice:
- Highly ordered, periodic structure
- Repeats in three dimensions
- Each lattice point is fixed
- Types include cubic, hexagonal, etc.
What is a Unit Cell?
The unit cell is the smallest part of a lattice that repeats in all directions to create the full crystal.
- Edge lengths: a, b, c
- Angles: α, β, γ
Remember: Crystal properties depend on the unit cell geometry.
Types of Unit Cells (Cubic System)
- Simple Cubic (SC): Atoms at corners. Coordination number: 6.
- Body-Centered Cubic (BCC): Corners + one in center. Coordination number: 8.
- Face-Centered Cubic (FCC): Corners + face centers. Coordination number: 12.
Calculations Involving Unit Cells
- Edge Length (a): Related to atomic radius.
- Density (ρ):
ρ = (Z × M) / (a³ × NA)
Where Z = number of atoms/unit cell, M = molar mass, a = edge length, NA = Avogadro’s number. - Packing Efficiency: Space occupied by atoms vs total volume.
Importance
- Determines mechanical, electrical properties
- Used in X-ray crystallography
- Vital in designing functional materials
Conclusion: Understanding crystal lattices and unit cells helps explain the structure and properties of solid materials, making them crucial in modern science and technology.
MCQ Quiz
- Which type of unit cell has atoms at corners and face centers?
A. SC B. BCC C. FCC D. None
✅ Explanation: FCC includes atoms at corners and all face centers. - The number of atoms per unit cell in BCC is:
A. 1 B. 2 C. 4 D. 6
✅ Explanation: BCC has 1 atom at center and 8 corners × 1/8 = 2 total.
True / False
- Crystal lattice is random and disordered.
❌ False – It is highly ordered and periodic. - Unit cells repeat in 3D to form the whole crystal.
✅ True - FCC unit cells have lower coordination number than BCC.
❌ False – FCC has 12, BCC has 8.

Pingback: Density of Unit Cell -
Pingback: Solid State Chemistry - CHEMASH