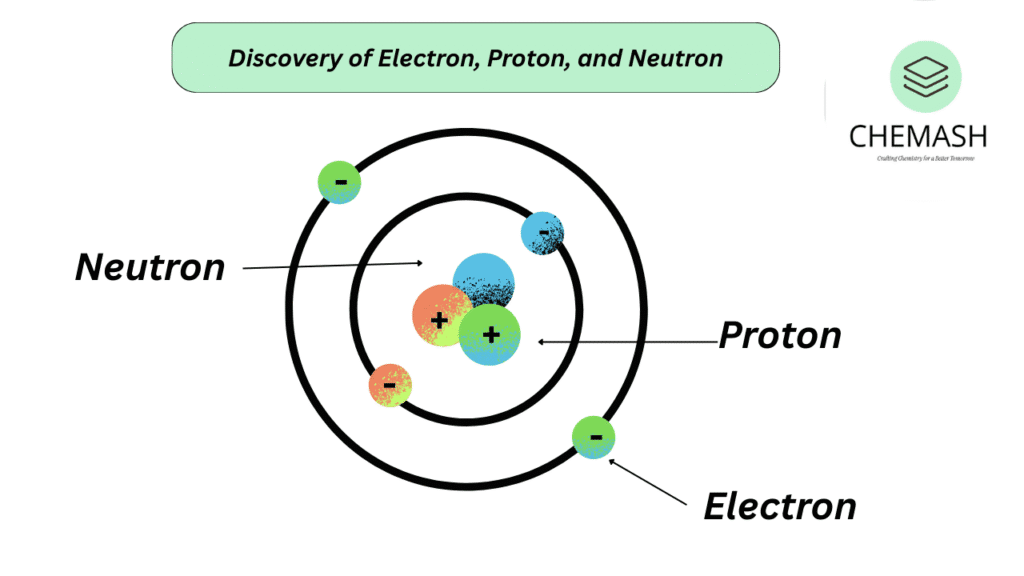
Discovery of Electron, Proton, and Neutron
1. Discovery of Electron (J.J. Thomson, 1897)
The electron was discovered by J.J. Thomson in 1897 during his experiments with cathode ray tubes. As a result, he observed that when high voltage was applied across two electrodes in a partially evacuated tube, a beam of negatively charged particles was emitted from the cathode. Moreover, this experiment provided the first evidence of subatomic particles. Discovery of Electron Proton and Neutron
Key Findings:
- The rays originated from the cathode and were deflected by electric and magnetic fields, therefore indicating negative charge.
- The charge-to-mass ratio (e/m) was the same for all metals and gases. Consequently, electrons were proven to be universal components of atoms.
- Thomson concluded that atoms contain tiny negatively charged subatomic particles called electrons. In other words, atoms were divisible into smaller parts.
Charge of Electron: -1.602 × 10⁻¹⁹ C
Mass of Electron: 9.1 × 10⁻³¹ kg
2. Discovery of Proton (Goldstein & Rutherford)
The proton was discovered by E. Goldstein in 1886 and later confirmed by Ernest Rutherford in 1919. Goldstein observed canal rays (positive rays) in discharge tubes with perforated cathodes. Furthermore, Rutherford confirmed that the hydrogen nucleus was a fundamental particle, naming it the proton. Thus, the idea of positively charged building blocks of matter was established.
Key Findings:
- Canal rays were positively charged particles moving opposite to electrons. Therefore, they proved the presence of positive charges inside atoms.
- Hydrogen nucleus is present in all atoms, proving protons as fundamental particles. In addition, this discovery laid the foundation of atomic structure.
Charge of Proton: +1.602 × 10⁻¹⁹ C
Mass of Proton: 1.67 × 10⁻²⁷ kg
3. Discovery of Neutron (James Chadwick, 1932)
The neutron was discovered by James Chadwick in 1932. He bombarded beryllium with alpha particles and, as a result, observed emission of neutral radiation that knocked protons from substances. Consequently, this confirmed the existence of a neutral particle inside the nucleus.
Key Findings:
- The radiation was unaffected by electric or magnetic fields; hence, it was neutral.
- Mass was similar to a proton; therefore, neutrons contributed significantly to atomic mass.
- Chadwick concluded these neutral particles were neutrons. Moreover, this explained isotopes of elements.
Charge of Neutron: 0 (Neutral)
Mass of Neutron: 1.675 × 10⁻²⁷ kg
Quick Recap:
– Electron → Discovered by J.J. Thomson (1897), negative charge.
– Proton → Observed by Goldstein (1886), confirmed by Rutherford (1919), positive charge.
– Neutron → Discovered by James Chadwick (1932), neutral.
Therefore, all three subatomic particles contributed to the modern atomic theory.
