Dynamic Nature of Equilibrium
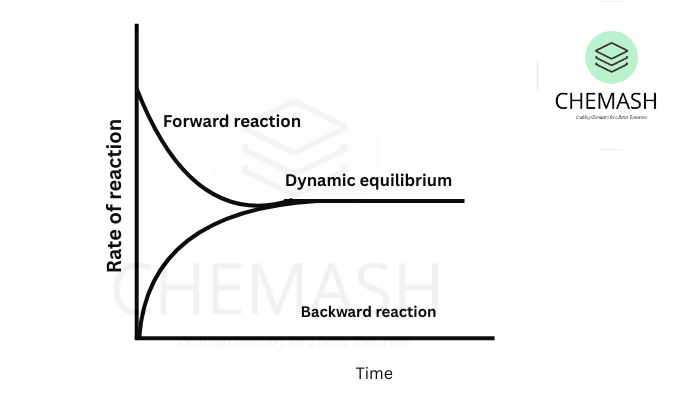
The dynamic nature of equilibrium in chemistry explains that while the concentrations of reactants and products remain constant over time, the system is not static. Instead, equilibrium is a dynamic process where forward and reverse reactions continue at equal rates.
Chemical equilibrium is often described as a state where the concentrations of reactants and products remain constant over time. However, it is crucial to understand that equilibrium is not a static or “dead” state, but rather a dynamic process.
Table of Contents
Introduction
Chemical equilibrium is not a static state but a dynamic process. Forward and reverse reactions occur simultaneously at equal rates, so concentrations remain constant over time.
Understanding the Dynamic Process
Consider a simple reversible reaction:
A + B ⇌ C + D
At equilibrium, forward and reverse reactions continue at equal rates, maintaining constant concentrations of reactants and products.
Why is Equilibrium Called Dynamic?
- Reactants continuously convert into products.
- Products simultaneously convert back to reactants.
- Balance is achieved without stopping reactions.
Microscopic Perspective
On a molecular scale, equilibrium is like a busy intersection where cars flow both ways equally—no net change in total traffic.
Significance of Dynamic Equilibrium
- Responsive System: Shifts with temperature, pressure, or concentration changes (Le Chatelier’s principle).
- Industrial Relevance: Helps optimize chemical yields.
- Biological Systems: Essential for processes like oxygen transport in blood.
Example: Esterification Reaction
Reversible reaction:
CH3COOH + C2H5OH ⇌ CH3COOC2H5 + H2O
At equilibrium, esterification and hydrolysis occur at equal rates, keeping concentrations constant.
Quiz: Test Your Understanding
- What does it mean when we say chemical equilibrium is dynamic?
- Why do concentrations remain constant at equilibrium?
- Does the forward reaction stop at equilibrium?
- Give an example of a reversible reaction with dynamic equilibrium.
Answers
- Both forward and reverse reactions occur at equal rates.
- Because the rates of both reactions are equal.
- No, forward reaction continues but at equal rate to reverse.
- The esterification of acetic acid and ethanol.
Summary
Dynamic equilibrium maintains stability by balancing forward and reverse reactions. This makes it fundamental in chemistry, biology, and industrial processes.
Wikipedia: Chemical Equilibrium
