Electrode Potential in Electrochemistry
Electrode potential is a fundamental concept in electrochemistry that explains the tendency of an electrode to gain or lose electrons when in contact with its own ions. It plays a vital role in redox reactions, electrochemical cells, and battery technologies.
Table of Contents
- What is Electrode Potential?
- Origin of Electrode Potential
- Standard Electrode Potential (E°)
- Standard Hydrogen Electrode (SHE)
- Measuring Electrode Potential
- Significance of Electrode Potentials
- Electrode Potential Table
- Cell Voltage Formula
- Applications
- MCQs
What is Electrode Potential?
Electrode potential is the voltage developed at the interface between an electrode and its ion solution due to electron transfer. It determines whether an electrode acts as an anode or cathode in a cell.
Origin of Electrode Potential
When a metal is dipped in its salt solution, metal atoms may lose electrons and become ions, generating potential difference due to accumulation of charge. This gives rise to electrode potential.
Standard Electrode Potential (E°)
Standard electrode potential is measured under standard conditions: 1M ion concentration, 1 atm pressure, and 25°C temperature. It’s denoted as E° and measured in volts (V).
Standard Hydrogen Electrode (SHE)
SHE is the reference electrode with a defined potential of 0V. It consists of platinum dipped in 1M H⁺ solution with hydrogen gas at 1 atm pressure bubbling over it. Other electrode potentials are measured relative to SHE.
Measuring Electrode Potential
To measure an electrode’s potential, connect it to the SHE and measure the voltage. If the electrode gains electrons, it’s reduction (positive potential), and if it loses electrons, it’s oxidation (negative potential).
Significance of Electrode Potentials
- Predict direction of redox reactions
- Helps in designing galvanic and electrolytic cells
- Used to calculate cell EMF
- Important in corrosion prevention and metallurgy
Standard Electrode Potential Table
| Electrode | Reaction | E° (V) |
|---|---|---|
| Fluorine | F₂ + 2e⁻ → 2F⁻ | +2.87 |
| Hydrogen | 2H⁺ + 2e⁻ → H₂ | 0.00 |
| Zinc | Zn²⁺ + 2e⁻ → Zn | −0.76 |
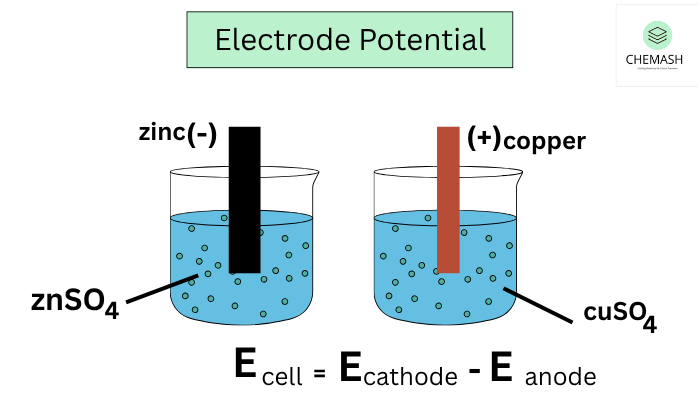
Cell Voltage Formula
EMF (E°cell) = E°cathode − E°anode
Example: For Zn–Cu cell: E°cell = (+0.34) − (−0.76) = +1.10 V
Applications
- Battery design (Li-ion, lead-acid, etc.)
- Electroplating and purification
- Corrosion protection
- Fuel cells and green energy storage
📚 Also read: Galvanic Cell – Working & Principle
MCQs
- Which electrode is used as a reference standard?
- a) Copper
- b) Zinc
- ✅ c) Hydrogen
- d) Silver
- What is the E° value of the Standard Hydrogen Electrode?
- a) +1.00 V
- b) −1.00 V
- ✅ c) 0.00 V
- d) +0.76 V
- Which electrode has the highest tendency to gain electrons?
- a) Zinc
- b) Copper
- ✅ c) Fluorine
- d) Hydrogen
