Electrode Potential
Electrode potentials is the potential difference that forms at the surface of an electrode when it contacts an electrolyte. It develops because the electrode tends to gain or lose electrons. This potential is measured in volts (V).
Types of Electrode Potentials
- Standard Electrode Potentials (E°): Measured under standard conditions (1 M, 1 atm, 25 °C) relative to the Standard Hydrogen Electrode (SHE).
- Single Electrode Potentials: The potential of an individual electrode compared to a reference electrode.
- Cell Potential (EMF): The overall voltage of a galvanic cell, equal to the difference between the electrode potentials of two electrodes.
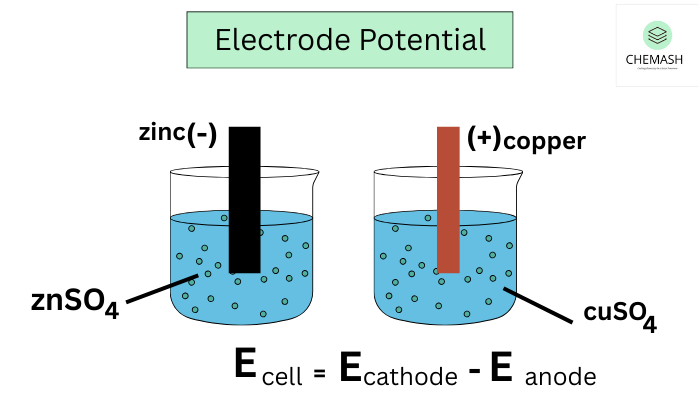
How Electrode Potentials Arises
When a metal contacts an electrolyte that contains its ions, electrons either move into the solution or return to the metal. This electron transfer separates charge at the interface and creates the electrode potentials.
Standard Hydrogen Electrode (SHE)
The SHE serves as the universal reference electrode. It consists of platinum in contact with 1 M H⁺ solution and hydrogen gas at 1 atm, maintained at 25 °C. By definition, its potential equals 0.00 V. Scientists use the SHE to measure all standard electrodes potentials.
Factors Affecting
- Ion concentration in the electrolyte
- Temperature
- Nature of the electrode material
- Pressure for gases involved
Significance
- Determines the direction of electron flow in electrochemical cells
- Predicts whether redox reactions occur spontaneously
- Helps calculate cell voltage for batteries and fuel cells
- Plays a key role in corrosion studies, electroplating, and electrolysis
Quiz
- What is electrodes potential?
- What are the standard conditions for measuring E°?
- What is the role of the Standard Hydrogen Electrode?
- List two factors that affect electrodes potential.
- Why is electrodes potential important for predicting spontaneity?
Answers & Explanation
- The voltage that develops at the electrode–electrolyte interface due to redox reactions.
- Standard conditions: 1 M, 1 atm, 25 °C.
- The SHE acts as the reference electrode with potential defined as 0.00 V.
- Ion concentration and temperature (others possible).
- It shows the tendency to gain or lose electrons and predicts spontaneity.
FAQ
Q1. How do you measure single electrode potential?
You connect the electrode to a reference electrode (like SHE or saturated calomel electrode) and measure the potential difference.
Q2. Can electrode potential change with conditions?
Yes. Changes in concentration, temperature, or pressure alter the electrode potential. The Nernst equation describes this relationship.
References
Electrode potential — Wikipedia
Nernst Equation — CHEMASH
