Empirical and Molecular Formulas
The empirical and molecular formulas are vital in representing the composition of chemical compounds. They help in understanding the relative and actual number of atoms of each element in a compound. The distinction between them is crucial for chemical calculations and formula derivation in stoichiometry. Empirical and Molecular Formula
Empirical Formula
The empirical formula of a compound shows the simplest whole number ratio of atoms of each element in the compound. It does not necessarily represent the actual number of atoms, just the ratio.
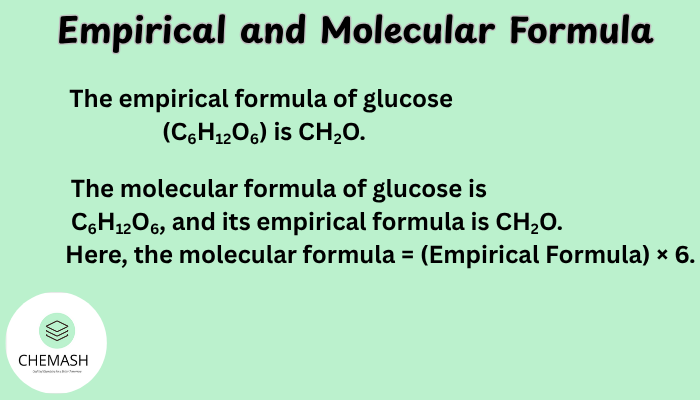
Example: The empirical formula of glucose (C6H12O6) is CH2O.
Molecular Formula
The molecular formula represents the actual number of atoms of each element in a molecule of the compound. It is a whole-number multiple of the empirical formula.
Example: The molecular formula of glucose is C6H12O6, and its empirical formula is CH2O. Here, the molecular formula = (Empirical Formula) × 6.
Formula Relationship
The relationship between molecular and empirical formula is:
Molecular Formula = n × Empirical Formula
where n is an integer and calculated as:
n = (Molar Mass of compound) / (Empirical Formula Mass)
Comparison Table
| Property | Empirical Formula | Molecular Formula |
|---|---|---|
| Represents | Simplest whole number ratio | Actual number of atoms |
| Uniqueness | May represent multiple compounds | Unique for a compound |
| Determination | From elemental analysis | From molar mass and empirical formula |
Example Problem
Given: A compound contains 40% Carbon, 6.7% Hydrogen, and 53.3% Oxygen. Its molar mass is 180 g/mol. Find its molecular formula.
- Assume 100 g sample → C = 40 g, H = 6.7 g, O = 53.3 g
- Moles: C = 40 / 12 = 3.33, H = 6.7 / 1 = 6.7, O = 53.3 / 16 = 3.33
- Divide by smallest: C = 1, H = 2, O = 1 → Empirical formula = CH2O
- Empirical mass = 12 + 2×1 + 16 = 30 g/mol
- n = 180 / 30 = 6 → Molecular formula = C6H12O6
Quiz
- The empirical formula of C4H8 is:
a) C4H8
b) CH2
c) C2H4
d) CH
Answer: b)
Explanation: Divide subscripts by 4 → CH2 is the simplest whole number ratio. - If a compound has empirical formula CH and molar mass 78, what is its molecular formula?
a) C2H2
b) C3H3
c) C6H6
d) C4H4
Answer: c)
Explanation: CH = 13 g/mol. 78 / 13 = 6 → C6H6. - Which of the following cannot be an empirical formula?
a) CH
b) CH2O
c) C2H6
d) NH2
Answer: c)
Explanation: C2H6 can be simplified to CH3. - Which technique helps determine empirical formula?
a) X-ray crystallography
b) Combustion analysis
c) Spectroscopy
d) Colorimetry
Answer: b)
Explanation: Combustion analysis provides elemental composition percentages.
Next Up: Stoichiometry and Calculations → Understand mole concepts and chemical equations.
