Enantiomers – Definition, Properties, Examples & Chirality
Enantiomers are a type of stereoisomer that exist as non-superimposable mirror images of each other. They have the same molecular formula and connectivity of atoms but differ in spatial arrangement around a chiral center.
What Makes a Molecule Chiral?
A molecule becomes chiral when it contains a chiral carbon (stereocenter) attached to four different substituent groups. Such molecules do not possess a plane of symmetry and exist in two mirror image forms — enantiomers.
Characteristics of Enantiomers
- Identical physical properties (melting point, boiling point, density, solubility).
- Differ in optical activity — one rotates plane-polarized light to the right, the other to the left.
- Same chemical properties except when reacting with chiral reagents or in biological systems.
- One enantiomer can be pharmacologically active while the other may be inactive or harmful.
Important: Enantiomers always occur in pairs — like right-hand and left-hand gloves.
Optical Activity – d & l / (+) & (–)
- (+) or d-enantiomer ⇒ Rotates plane polarized light to the **right** (dextrorotatory)
- (–) or l-enantiomer ⇒ Rotates plane polarized light to the **left** (levorotatory)
Note: Optical rotation (d/l or +/–) is **not** the same as R/S configuration.
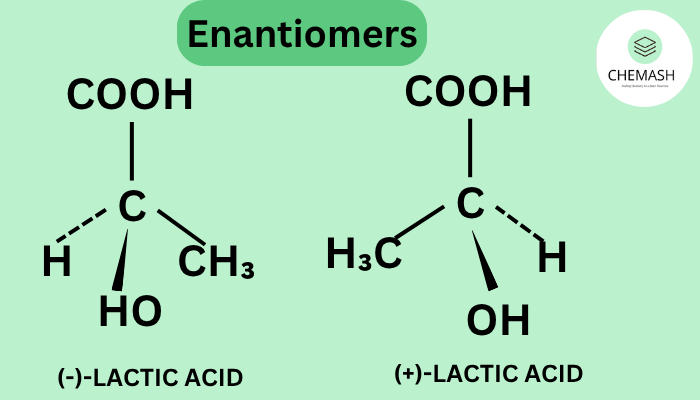
Example of Enantiomers
Lactic Acid (CH₃–CH(OH)–COOH) exists as two enantiomers:
- (R)-lactic acid
- (S)-lactic acid
In the pharmaceutical industry, only one enantiomer may show desired biological activity — making stereochemistry critical in drug development.
Difference Between Enantiomers and Diastereomers
| Enantiomers | Diastereomers |
|---|---|
| Non-superimposable mirror images | Not mirror images |
| Always occur in pairs | Can be two or more |
| Similar physical properties | Different physical properties |
| Opposite optical rotation | No fixed relation to optical rotation |
FAQs
Q1. What causes enantiomerism?
Presence of a chiral carbon attached to four different groups.
Q2. How do enantiomer differ physically?
They differ only in the direction of optical rotation.
Q3. Can enantiomer have different scents or tastes?
Yes — due to interactions with chiral biological receptors. (Example: Carvone – spearmint vs. caraway aroma.)
Quick Quiz (Check Your Understanding)
1. Enantiomers are:
- A) Superimposable mirror images
- B) Non-superimposable mirror images ✔️
- C) Geometric isomers
2. Enantiomers differ most notably in:
- A) Boiling point
- B) Melting point
- C) Optical activity ✔️

Pingback: Diastereomers - CHEMASH