Formation of Enolates
Formation of enolates plays a crucial role in organic chemistry. In particular, it explains the behavior of aldehydes and ketones in many important reactions. Moreover, enolates act as powerful nucleophiles. As a result, they participate actively in reactions such as aldol condensation, Claisen condensation, and alkylation reactions.
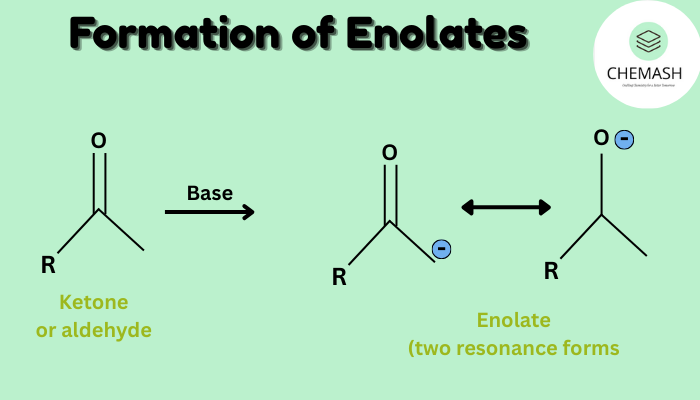
Table of Contents
- What is an Enolate?
- Role of α-Hydrogen
- Mechanism of Enolate Formation
- Enolate Formation in Acidic Medium
- Enolate Formation in Basic Medium
- Factors Affecting Enolate Formation
- Examples
- Exam Importance
- MCQs
- FAQs
- Reference Links
What is an Enolate?
An enolate ion forms when a base removes an α-hydrogen from a carbonyl compound. Consequently, this process generates a resonance-stabilized anion.
Furthermore, enolates exist in two important resonance forms:
- Negative charge on oxygen (major contributor)
- Negative charge on α-carbon
Role of α-Hydrogen
The α-hydrogen refers to the hydrogen atom attached to the carbon adjacent to the carbonyl group. Therefore, its presence becomes essential for enolate formation.
α-hydrogen shows acidic character because:
- The resulting enolate ion gains resonance stabilization
- The carbonyl group pulls electrons due to its –I effect
In contrast, compounds without α-hydrogen, such as benzaldehyde, do not form enolates.
Mechanism of Enolate Formation
Step 1: Abstraction of α-Hydrogen
First, a strong base removes the α-hydrogen from the carbonyl compound. As a result, a carbanion begins to form.
Step 2: Formation of Enolate Ion
Next, electron movement creates a delocalized enolate ion. Moreover, resonance between oxygen and carbon stabilizes this ion.
Subsequently, the enolate behaves as a nucleophile and attacks suitable electrophiles in further reactions.
Enolate Formation in Acidic Medium
In acidic conditions, enolate formation proceeds through enol formation rather than direct ion generation.
- First, the carbonyl oxygen gets protonated
- Then, a weak base such as water removes the α-hydrogen
- Finally, an enol (C=C–OH) forms
Therefore, acidic conditions favor enol formation instead of full enolate ion formation.
Enolate Formation in Basic Medium
In basic medium, enolates form directly and efficiently. Consequently, this pathway dominates in many carbon–carbon bond reactions.
- Strong bases like OH⁻, LDA, or NaOEt remove α-hydrogen
- The reaction produces a stable enolate ion
- These enolates then participate in aldol and Claisen condensations
Factors Affecting Enolate Formation
- Acidity of α-hydrogen
- Strength and nature of the base
- Solvent used in the reaction
- Substituents attached to the carbonyl carbon
Examples of Enolate Formation
For example, acetone reacts with NaOH to form an enolate ion. Subsequently, this enolate takes part in aldol condensation.
Similarly, ethyl acetoacetate forms highly stabilized enolates because it contains two carbonyl groups.
Importance for Class 12 & NEET
Understanding enolate formation helps students master several key reactions. Therefore, this topic holds great importance in exams.
- Aldol condensation
- Crossed aldol reactions
- Claisen condensation
- Carbon–carbon bond formation
As a result, examiners frequently ask mechanism-based questions from this topic in Class 12 board exams and NEET.
MCQs on Formation of Enolates
Q1. Which hydrogen is removed during enolate formation?
- A. β-hydrogen
- B. α-hydrogen ✅
- C. Aromatic hydrogen
- D. Terminal hydrogen
Answer: α-hydrogen because resonance stabilization follows.
Frequently Asked Questions (FAQs)
Why are enolates good nucleophiles?
Enolates act as strong nucleophiles because their negative charge spreads over carbon and oxygen through resonance.
Do all carbonyl compounds form enolates?
No. Only carbonyl compounds containing at least one α-hydrogen can form enolates.
