Enthalpy and Heat Changes
In thermodynamics, understanding the flow and transformation of energy is crucial. One of the most important energy terms used to quantify heat change during a chemical or physical process is enthalpy (H).
What is Enthalpy?
Enthalpy (H) is a thermodynamic quantity equivalent to the total heat content of a system. It is defined as:
H = U + PV
Where:
H = Enthalpy
U = Internal energy of the system
P = Pressure
V = Volume
Enthalpy is a state function, meaning it depends only on the current state of the system, not the path taken to reach that state.
Heat Changes and Enthalpy
At constant pressure, the heat absorbed or released by a system is equal to the change in enthalpy:
ΔH = qp
Where:
ΔH = Change in enthalpy
qp = Heat exchanged at constant pressure
Types of Enthalpy Changes
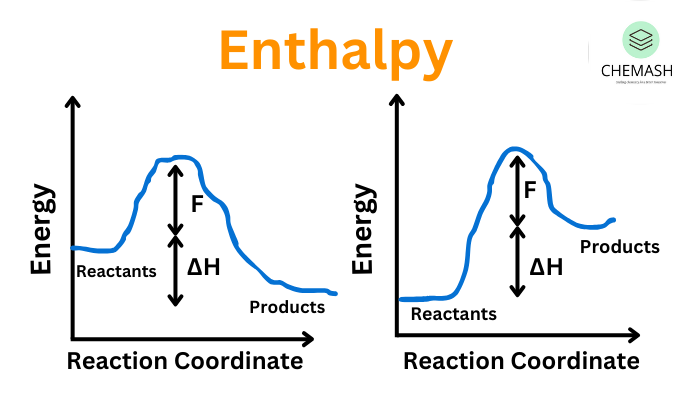
- Exothermic Process: Releases heat; ΔH < 0 (Example: Combustion of fuels)
- Endothermic Process: Absorbs heat; ΔH > 0 (Example: Photosynthesis)
Important Enthalpy Changes in Chemistry
- Enthalpy of Formation (ΔHf): Heat change when 1 mole of compound forms from its elements in standard states.
- Enthalpy of Combustion (ΔHc): Heat released when 1 mole of substance burns in oxygen.
- Enthalpy of Neutralization: Heat change when acid reacts with base to form 1 mole of water.
- Enthalpy of Fusion: Heat required to convert 1 mole of solid to liquid at melting point.
- Enthalpy of Vaporization: Heat required to convert 1 mole of liquid to gas at boiling point.
- Enthalpy of Sublimation: Heat required to convert 1 mole of solid to gas without becoming liquid.
Standard Enthalpy Change
Standard enthalpy changes (ΔH°) refer to changes under standard conditions: 298 K (25°C), 1 atm pressure, and 1 M concentration for solutions.
Example: The standard enthalpy of formation of water is -285.8 kJ/mol, meaning that 285.8 kJ of heat is released when 1 mole of water forms from hydrogen and oxygen at standard conditions.
Importance of Enthalpy
- Helps predict the heat exchange in chemical reactions.
- Used in designing industrial processes (e.g., Haber process).
- Essential in determining energy efficiency of fuels.
- Useful in biochemistry for studying metabolic energy changes.
Conclusion: Enthalpy is a fundamental thermodynamic quantity that provides insight into energy flow in chemical and physical changes. By understanding enthalpy changes, chemists can determine whether a process is feasible, how much energy is involved, and how it can be optimized for real-world applications.
MCQs with Answers & Explanations
Q1. Which of the following best defines enthalpy (H)?
a) Heat absorbed or released at constant pressure
b) Heat absorbed or released at constant volume
c) Energy stored in bonds only
d) Energy required to break bonds only
Answer: a) Heat absorbed or released at constant pressure
Explanation: Enthalpy represents the total heat content of a system at constant pressure.
Q2. An exothermic reaction has:
a) ΔH > 0
b) ΔH < 0
c) ΔH = 0
d) No change in enthalpy
Answer: b) ΔH < 0
Explanation: Negative ΔH means heat is released to the surroundings.
Q3. Which of the following is a state function?
a) Heat
b) Work
c) Enthalpy
d) Path taken
Answer: c) Enthalpy
Explanation: Enthalpy depends only on initial and final states, not the path.
Q4. Units of enthalpy in SI system are:
a) Joule (J)
b) Calorie (cal)
c) kJ mol⁻¹
d) Both a and c
Answer: d) Both a and c
Explanation: Enthalpy is expressed in joules or kilojoules per mole.
Q5. Enthalpy change for an endothermic process is:
a) Negative
b) Positive
c) Zero
d) Infinite
Answer: b) Positive
Explanation: In endothermic processes, the system absorbs heat from surroundings.
True/False
- Enthalpy is a state function. ✅ True – It depends only on the initial and final states.
- In an exothermic reaction, ΔH is positive. ❌ False – It is negative.
- Heat capacity and enthalpy are the same. ❌ False – Heat capacity is the amount of heat needed to raise temperature, enthalpy is total heat content.
- Enthalpy change can be measured directly. ❌ False – It is calculated from measurable quantities like heat flow at constant pressure.
- Enthalpy change of fusion is always negative. ❌ False – It is positive because heat is absorbed.
Short Quiz
Fill in the blanks:
- Enthalpy change (ΔH) = ______ – ______ (products – reactants).
- A positive ΔH indicates a/an ______ reaction.
- The unit of enthalpy in the SI system is ______.
- The symbol for enthalpy is ______.
- At constant pressure, ΔH equals ______.
FAQs
Q1: What is enthalpy in thermodynamics?
Enthalpy is the total heat content of a system at constant pressure, represented by H.
Q2: How is enthalpy change calculated?
ΔH = H(products) – H(reactants).
Q3: What are the types of enthalpy changes?
Common types include enthalpy of reaction, formation, combustion, fusion, and vaporization.
Q4: How does enthalpy differ from internal energy?
Enthalpy includes internal energy plus the product of pressure and volume (H = U + PV).
Q5: Why is ΔH negative for exothermic reactions?
Because the system releases heat to the surroundings, reducing its heat content.

Pingback: Hess’s Law - CHEMASH