Equilibrium and Free Energy
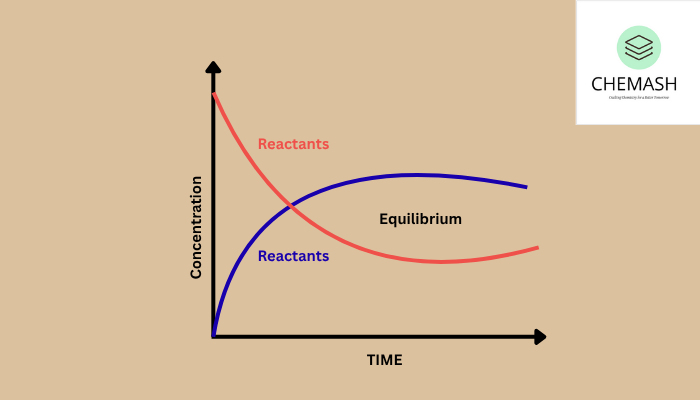
In thermodynamics, equilibrium refers to a state where the macroscopic properties of a system remain constant over time. At chemical equilibrium, the forward and reverse reactions occur at the same rate, and the concentrations of reactants and products remain unchanged.
Free energy, especially Gibbs Free Energy (G), plays a crucial role in determining whether a reaction will proceed spontaneously and what the position of equilibrium will be.

Gibbs Free Energy (ΔG)
The Gibbs Free Energy is a thermodynamic quantity that combines enthalpy (ΔH), entropy (ΔS), and temperature (T) to predict the spontaneity of a process.
ΔG = ΔH − TΔS
- ΔG < 0: Reaction is spontaneous (products favored)
- ΔG > 0: Reaction is non-spontaneous (reactants favored)
- ΔG = 0: System is at equilibrium
Key Insight: At equilibrium, a system’s free energy is at its minimum value.
Free Energy and Equilibrium Constant (K)
The relationship between the standard free energy change (ΔG⁰) and the equilibrium constant (K) is given by:
ΔG⁰ = −RT ln K
Where:
ΔG⁰ = Standard Gibbs Free Energy change
R = 8.314 J/mol·K
T = Temperature in Kelvin
K = Equilibrium constant
Interpretation:
- K > 1: Products favored, ΔG⁰ < 0 (spontaneous)
- K < 1: Reactants favored, ΔG⁰ > 0 (non-spontaneous)
- K = 1: ΔG⁰ = 0 (balanced equilibrium)
Real-World Example: In biological systems, reactions with ΔG⁰ > 0 can still occur if coupled with reactions having large negative ΔG⁰, like ATP hydrolysis.
Dynamic Nature of Equilibrium
- No net change in reactant/product concentrations
- Free energy is minimized
- Forward and reverse rates are equal
As a reaction approaches equilibrium, ΔG decreases and eventually becomes zero.
Summary
- ΔG predicts reaction spontaneity
- At equilibrium, ΔG = 0
- K relates directly to ΔG⁰
- Important in kinetics, equilibrium, and biochemistry
Conclusion: Understanding equilibrium and free energy helps explain how and why reactions occur.
MCQ Quiz
- Which condition indicates equilibrium?
ΔG > 0
ΔG < 0
ΔG = 0 ✅ - Relation between ΔG° and K?
ΔG° = RT ln K
ΔG° = −RT ln K ✅
ΔG° = −R/T ln K - If K > 1:
Products favored ✅
Reactants favored - Which term represents disorder?
Enthalpy
Entropy ✅ - ATP hydrolysis has:
Positive ΔG°
Negative ΔG° ✅
True / False
- At equilibrium, forward and reverse rates are equal. – True ✅
- ΔG < 0 means a non-spontaneous reaction. – False ❌
- K < 1 means products are favored. – False ❌
FAQs
What is Gibbs Free Energy?
It’s the energy available to do work at constant temperature and pressure, combining enthalpy, entropy, and temperature.
Why is ΔG important?
It predicts if a reaction is spontaneous under given conditions.
Can non-spontaneous reactions occur?
Yes, if coupled with highly exergonic reactions like ATP hydrolysis.

Pingback: chemical-reactions-of-phenol