Fischer Projections (फिशर प्रोजेक्शन)
Short summary: Fischer projections are a 2D notation to represent stereochemistry of molecules (especially sugars and amino acids) with chiral centers drawn as a cross: horizontal bonds project out of the page (toward viewer) and vertical bonds go behind the page. Fischer प्रोजेक्शन विशेष रूप से कार्बोहाइड्रेट और एमिनो अम्लों की स्टीरीओरसायन दिखाने के लिए प्रयोग होती है।
Why use Fischer projections? (उद्देश्य)
- Simple 2-D way to compare stereochemistry of molecules with multiple chiral centers.
- Standard for carbohydrates (D/L assignment of sugars) and many textbooks.
Essential rules (नियम)
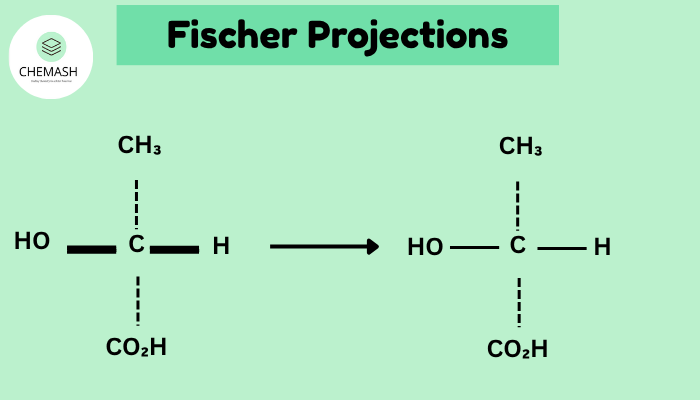
- Draw chiral carbon as the intersection (center) of a cross. Each intersection is a stereocenter.
- Horizontal lines = substituents coming out of the page (toward viewer). (होरिज़ॉन्टल बाहर की ओर)
- Vertical lines = substituents going behind the page (away from viewer). (वर्टिकल पीछे की ओर)
- Number carbons vertically with the most oxidized carbon (e.g., aldehyde carbon in sugars) at top for sugars convention.
- Rotation by 180° in plane is allowed (gives equivalent Fischer). Rotation by 90° in plane is NOT allowed (changes stereochemistry).
- To convert Fischer → wedge/dash or sawhorse, place horizontal groups toward the viewer and vertical groups away from the viewer when building 3D models.
Basic example: Glyceraldehyde (ग्लिसराल्डिहाइड)
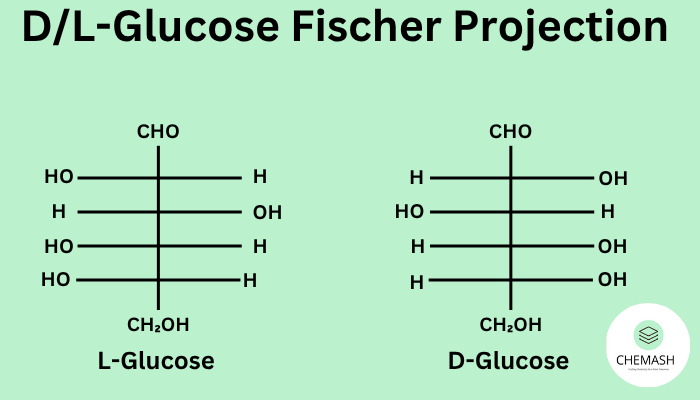
Glyceraldehyde has one stereocenter. Two enantiomers are conventionally labeled D- and L- by the configuration at that stereocenter in the Fischer projection (relative to D-glyceraldehyde).
D-Glyceraldehyde (Fischer)
CHO
|
H — C — OH
|
CH2OH
L-Glyceraldehyde (mirror)
CHO
|
OH — C — H
|
CH2OH
Note: In Fischer, if the OH on the stereocenter is to the right → D; to the left → L (for glyceraldehyde-type system).
Converting Fischer to wedge/dash (quick method)
- Identify the chiral carbon in Fischer.
- Draw the carbon center as tetrahedral: put horizontal substituents on wedge (toward), vertical substituents on dash (away) or vice versa depending on orientation.
- Assign priorities (Cahn-Ingold-Prelog) to get R/S if required — remember to correct if you had to invert while converting.
Important: Rotating a Fischer by 180° in the plane preserves stereochemistry; rotating by 90° in the plane inverts configuration (don’t do it unless you also invert again).
Common pitfalls (सामान्य भूलें)
- Mistaking horizontal for behind-the-plane — horizontal = outwards (toward viewer).
- Performing 90° rotations of the entire Fischer — this changes stereochemistry.
- For cyclic sugars (pyranoses/furanoses) Fischer is a projection of the open chain — ring forms use Haworth or chair conformations.
D/L vs R/S — short note
D/L is relative nomenclature based on glyceraldehyde reference (used for sugars & amino acids).
R/S is absolute assignment using Cahn–Ingold–Prelog priority rules. A molecule labeled D can be R or S; the systems are independent.
छोटी सारांश सारणी (Quick Rules — हिंदी)
- Horizontal बाहर → सामने। Vertical पीछे → पीछे।
- 180° planar rotation ठीक है; 90° planar rotation गलत है।
- ओएच दायीं ओर → D; बायीं ओर → L (sugars के लिए, तभी मान्य जब सामान्य sugar skeleton हो)।
Practice — MCQs, True/False, Fill-ups, Matching (with answers & explanations)
MCQs (बहुविकल्प)
- In a Fischer projection, horizontal bonds represent:
A) Bonds going behind plane
B) Bonds coming out of plane (toward viewer)
C) Bonds in plane
D) Aromatic bonds
Answer: B — Horizontal bonds project toward the viewer (out of page). - Which rotation of a Fischer projection does NOT change stereochemistry?
A) 90° in plane
B) 180° in plane
C) Any rotation is OK
D) 270° in plane
Answer: B — 180° (in-plane) is allowed and preserves stereochemistry. 90° is not allowed. - For glyceraldehyde, OH on right in Fischer =
A) L
B) D
C) R
D) S
Answer: B — D (by definition for glyceraldehyde-type reference).
True / False (सही / गलत)
- TF1: Vertical bonds in Fischer point away from the viewer. → True
- TF2: Rotating a Fischer by 90° in the plane keeps the same stereochemical configuration. → False
Fill in the blanks (रिक्त स्थान भरें)
- Horizontal lines in a Fischer projection point __________. → toward the viewer (out of plane)
- Rotation by __________ degrees in the plane preserves configuration. → 180°
Matching (मिलान करें)
| Column A | Match → Column B |
|---|---|
| Horizontal groups | → Out of page (toward) |
| Vertical groups | → Behind page (away) |
| 90° planar rotation | → Changes stereochemistry |
Short answers & explanations
Why is 90° rotation forbidden?
A 90° rotation swaps two substituents around each stereocenter relative to the viewer and will invert configuration — it is not an equivalent representation.
FAQs (अक्सर पूछे जाने वाले प्रश्न)
Q1: Can I rotate a Fischer projection by 180° and still have the same molecule?
A: Yes — 180° rotation in the plane yields an equivalent Fischer projection.
Q2: How do I convert Fischer to wedge/dash reliably?
A: Treat horizontals as wedges (coming out) and verticals as dashes (going back), then redraw the tetrahedron and assign priorities for R/S if needed.
Q3: Is Fischer used for cyclic molecules?
A: Fischer primarily represents open-chain stereochemistry (sugars). For rings, use Haworth or chair conformations which better reflect ring geometry.
Q4: How does D/L relate to R/S?
A: D/L is relative (glyceraldehyde reference) used in biochemistry; R/S is absolute (CIP rules). They do not directly map one-to-one.
Author: CHEMASH • Updated: Nov 2025
