Gibbs Free Energy
Gibbs Free Energy (G) is a thermodynamic potential that measures the maximum useful work obtainable from a process at constant temperature and pressure.
Definition and Formula
Gibbs Free Energy is defined as:
G = H – T × S
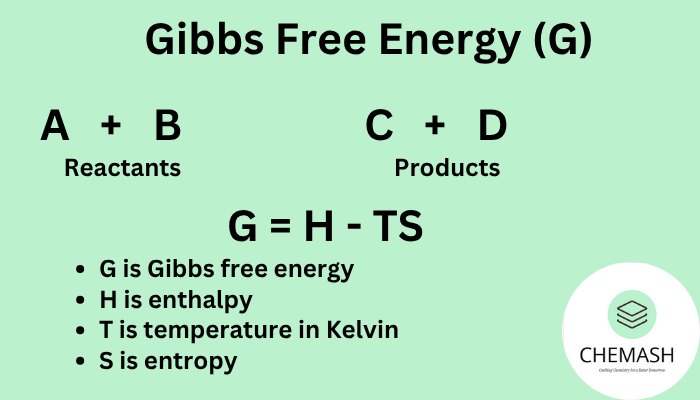
Where:
G = Gibbs free energy (Joules)
H = Enthalpy (heat content, J)
T = Temperature (Kelvin)
S = Entropy (disorder, J/K)
Significance of Gibbs Free Energy Change (ΔG)
- ΔG < 0: Spontaneous reaction (exergonic).
- ΔG = 0: System at equilibrium.
- ΔG > 0: Non-spontaneous reaction (endergonic).
Relationship with Equilibrium Constant
ΔG° relates to equilibrium constant (K) as:
ΔG° = -RT ln K
R = 8.314 J/mol·K, T = Temperature in Kelvin, K = Equilibrium constant.
Learn more about Thermodynamics basics and Wikipedia on Gibbs Free Energy.
Applications
- Predicting spontaneity of chemical reactions.
- Determining equilibrium positions.
- Calculating maximum work obtainable.
- Explaining biological processes like ATP hydrolysis.
Quiz
- Which term in the Gibbs frees energy (G)equation represents disorder?
a) G b) H c) T d) S
✅ Answer: d) Entropy
Explanation: Entropy (S) represents disorder. - A reaction with ΔG < 0 is:
a) Non-spontaneous b) Equilibrium c) Spontaneous d) Endergonic
✅ Answer: c) Spontaneous
Explanation: Negative ΔG = spontaneous. - At equilibrium, ΔG is:
a) Negative b) Positive c) Zero d) Undefined
✅ Answer: c) Zero
Explanation: No net change at equilibrium. - Which equation relates ΔG° and equilibrium constant?
a) ΔG° = RT ln K b) ΔG° = -RT ln K c) ΔG° = T/S d) ΔG° = H – TS
✅ Answer: b) ΔG° = -RT ln K
Explanation: Standard free energy links to K.
FAQ
Q1: What does negative Gibbs frees energy mean?
Answer: It means the process is spontaneous and releases energy.
Q2: Can ΔG predict equilibrium?
Answer: Yes, at equilibrium ΔG = 0, meaning no further net change.
Q3: How is Gibbs Frees Energy used in biology?
Answer: It explains processes like ATP hydrolysis, enzyme action, and metabolism.
Up Next: Entropy — Understanding disorder and spontaneity in thermodynamics.
