Group 16: Oxygen Family (Chalcogens)
Group 16 elements of the periodic table are known as the Oxygen Family or Chalcogens. This group includes Oxygen (O), Sulfur (S), Selenium (Se), Tellurium (Te), and Polonium (Po). These elements show a wide range of physical and chemical properties, ranging from non-metallic (O, S) to metalloid (Te) to metallic (Po).
Electronic Configuration and General Features
The general electronic configuration of Group 16 elements is ns² np⁴. They have six valence electrons and tend to gain or share electrons to achieve a stable octet:
- Common oxidation states are –2, +4, and +6.
- Oxygen primarily shows –2 oxidation state.
- The tendency to form –2 ions decreases down the group due to increasing atomic size and metallic character.
Physical Properties
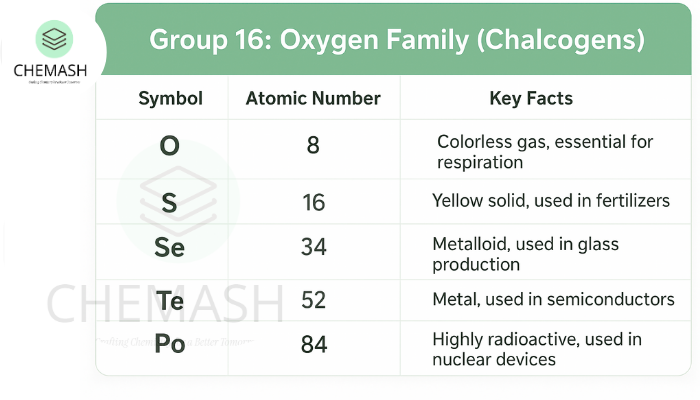
- Oxygen is a colorless, odorless gas; sulfur is a yellow solid.
- Melting and boiling points increase down the group.
- Electrical conductivity increases down the group (Po is metallic).
- Density increases from oxygen to polonium.
- Allotropy is common (O₂, O₃; rhombic and monoclinic sulfur).
Chemical Properties
- Oxidation States: Oxygen shows –2; others show –2, +4, +6 (due to d-orbitals).
- Reactivity with Hydrogen: Form hydrides (H₂E), acidic in nature, thermal stability decreases down the group.
- Reaction with Metals: Form oxides and sulfides (e.g., Na₂O, FeS).
- Reaction with Oxygen: Form oxides like SO₂, SeO₂ (acidic in nature).
- Electronegativity: Decreases down the group; oxygen is the second most electronegative element.
Important Compounds and Uses
- Oxygen (O₂): Essential for respiration and combustion; used in welding and life support systems.
- Ozone (O₃): Used in water purification and as a disinfectant.
- Sulfuric acid (H₂SO₄): Industrially important; used in fertilizers, batteries, and chemicals.
- Hydrogen sulfide (H₂S): Found in natural gas; used in chemical analysis.
- Tellurium compounds: Used in semiconductors and solar cells.
- Polonium: Highly radioactive; used in nuclear batteries and anti-static devices.
Trends in the Group
- Atomic and ionic radii increase from O to Po.
- Ionization energy decreases down the group.
- Electronegativity decreases from O to Po.
- Metallic character increases down the group.
- Acidity of hydrides decreases and thermal stability decreases.
- Tendency to form –2 ions decreases from O to Po.
MCQs: Test Your Knowledge
- The general electronic configuration of Group 16 elements is:
- a) ns² np³
- b) ns² np⁴ ✅
- c) ns² np⁵
- Which element of Group 16 shows only –2 oxidation state?
- a) Sulfur
- b) Oxygen ✅
- c) Selenium
- Which compound of sulfur is most industrially important?
- a) H₂S
- b) SO₂
- c) H₂SO₄ ✅
FAQs
Q1. Why is oxygen more electronegative than sulfur?
Oxygen has a smaller atomic size and higher effective nuclear charge compared to sulfur, making it more electronegative.
Q2. Why does metallic character increase down Group 16?
As we move down the group, atomic size increases and ionization energy decreases, leading to metallic behavior in heavier elements.
Q3. Which is the most stable hydride of Group 16?
H₂O (water) is the most stable hydride due to strong hydrogen bonding.
Q4. What are the oxidation states of sulfur?
Sulfur shows –2, +4, and +6 oxidation states due to availability of d-orbitals.
Read more about Group 15: Nitrogen Family
