Haloarenes
Table of Contents
- Introduction
- Structure & Bonding
- Nomenclature
- Methods of Preparation
- Physical Properties
- Chemical Properties
- Reactivity vs Haloalkanes
- Environmental Significance
- Quiz
- Fill in the Blanks
- FAQ
Introduction
Haloarenes are aromatic compounds in which one or more hydrogen atoms of an aromatic ring (usually benzene) are replaced by halogen atoms (F, Cl, Br, I). A common example is chlorobenzene (C₆H₅Cl).
Structure and Bonding
- The carbon–halogen (C–X) bond in haloarenes has partial double bond character due to resonance.
- The halogen’s lone pair delocalizes into the aromatic π-system, stabilizing the molecule.
- This makes the C–X bond stronger and less reactive toward nucleophiles.
Nomenclature
Naming follows IUPAC rules. For mono-substituted compounds, the halogen is named as a prefix.
- C₆H₅Cl → Chlorobenzene
- C₆H₄Cl₂ → 1,2-Dichlorobenzene (ortho), 1,3-Dichlorobenzene (meta), 1,4-Dichlorobenzene (para)
Methods of Preparation
- Direct Halogenation: Benzene + Cl₂ → Chlorobenzene (in presence of FeCl₃)
- Sandmeyer Reaction: C₆H₅N₂⁺Cl⁻ + CuCl → C₆H₅Cl + N₂
- Gattermann Reaction: C₆H₅N₂⁺Cl⁻ + Cu/HCl → C₆H₅Cl
Physical Properties
- Less polar than alkyl halides.
- Insoluble in water, soluble in organic solvents.
- Boiling point increases with molecular mass.
Chemical Properties
1. Nucleophilic Substitution
Haloarenes are less reactive due to resonance stabilization and partial double bond character.
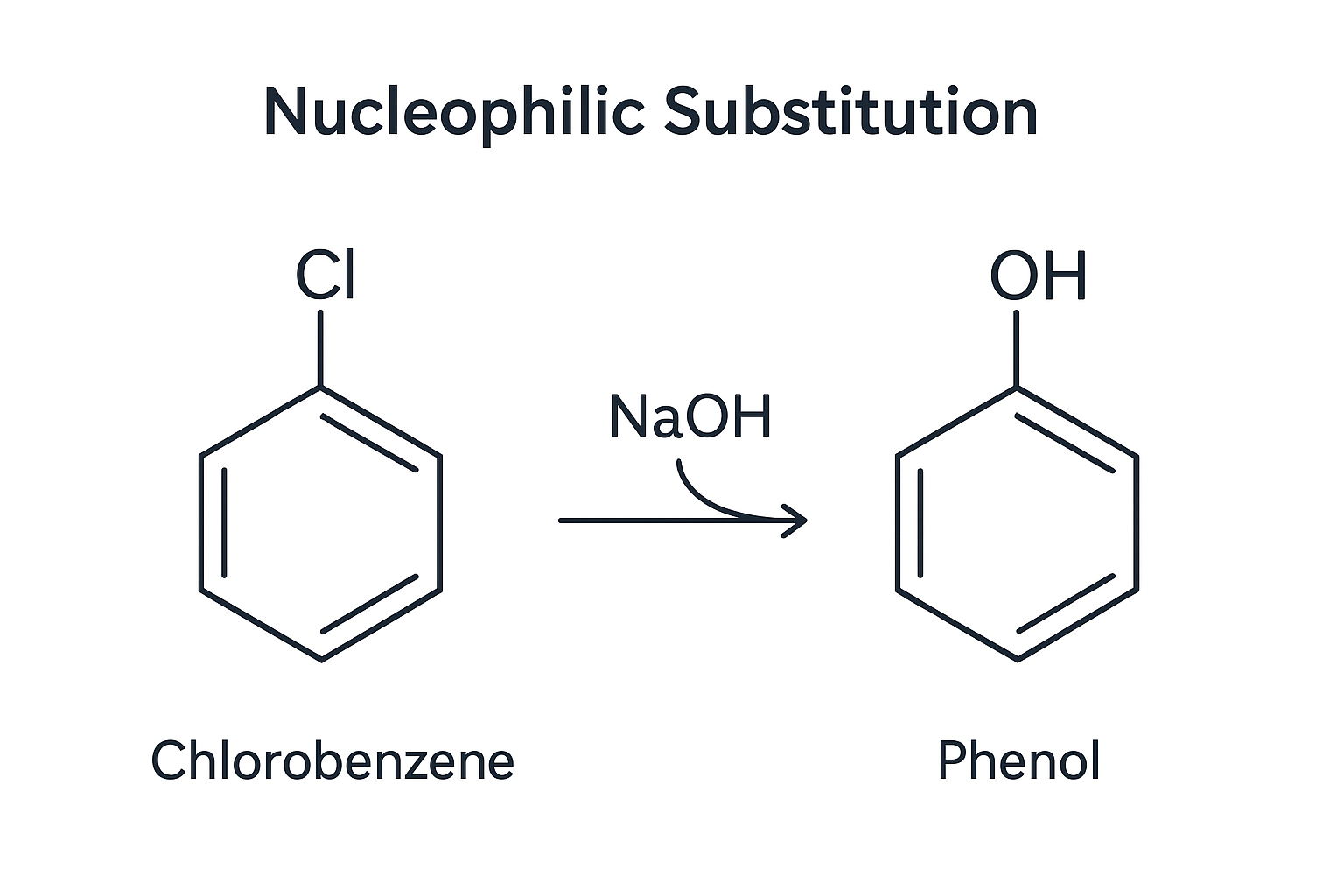
Example: C₆H₅Cl + NaOH (300°C, 200 atm) → C₆H₅OH
2. Electrophilic Substitution
Halogen shows –I effect (electron-withdrawing) and +M effect (electron-donating). This deactivates the ring but directs substitution to ortho & para positions.
- Nitration: C₆H₅Cl + HNO₃ → o-/p-Chloronitrobenzene
- Sulfonation: C₆H₅Cl + H₂SO₄ → o-/p-Chlorobenzenesulfonic acid
- Friedel-Crafts: Possible but slower than benzene
Reactivity Difference from Haloalkanes
- Haloalkanes: C–X bond more reactive (no resonance).
- Haloarenes: Resonance stabilizes C–X bond, making substitution difficult.
Environmental Significance
- Some haloarenes (e.g., DDT) are persistent pollutants.
- They accumulate in food chains → long-term toxicity.
- Strict regulations ensure controlled use & disposal.
Did you know? Chlorobenzene is used in the manufacture of dyes, pesticides, and pharmaceuticals!
Quiz
1. Which halogenated compound is the simplest haloarene?
✅ Chlorobenzene
2. Which reaction involves diazonium salts?
✅ Sandmeyer Reaction
3. Why are haloarenes less reactive than haloalkanes?
✅ Due to resonance stabilization
Fill in the Blanks
- Haloarenes are aromatic compounds containing __________ atoms. (Answer: halogen)
- In haloarenes, the C–X bond shows __________ character. (Answer: partial double bond)
- Chlorobenzene is prepared from benzene by __________ reaction. (Answer: halogenation)
Frequently Asked Questions
Q1. What are haloarenes?
Aromatic compounds where one or more hydrogens of benzene are replaced by halogens.
Q2. Why is the C–X bond in haloarenes strong?
Resonance gives it partial double bond character.
Q3. Are haloarenes soluble in water?
No, they are insoluble in water but dissolve in organic solvents.
Q4. Why are haloarenes environmentally significant?
Many are toxic, persistent, and bioaccumulative in ecosystems.

Pingback: Haloalkanes (Alkyl Halides) - Chemistry Notes - CHEMASH