Hess’s Law of Constant Heat Summation
Hess’s Law states that the total enthalpy change for a chemical reaction is the same, no matter how many steps the reaction is carried out in.
Explanation
Hess’s Law is based on the principle that enthalpy is a state function. The enthalpy change (ΔH) depends only on the initial and final states of the system — not on the intermediate path or steps taken.
Therefore, whether a reaction occurs in a single step or multiple steps, the overall enthalpy change remains the same.
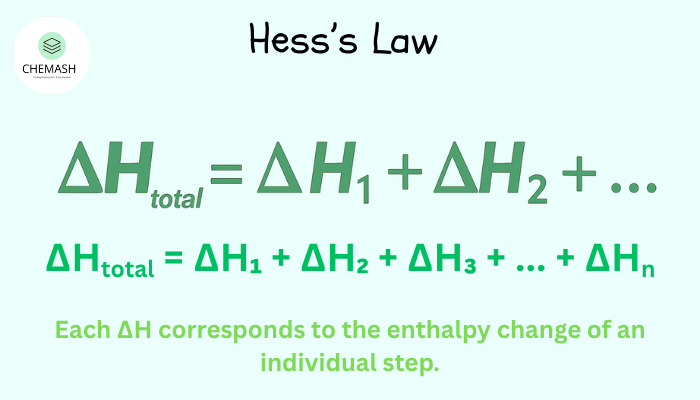
Mathematical Expression
If a reaction can be expressed as a sum of steps:
ΔHtotal = ΔH₁ + ΔH₂ + ΔH₃ + … + ΔHn
Each ΔH corresponds to the enthalpy change of an individual step.
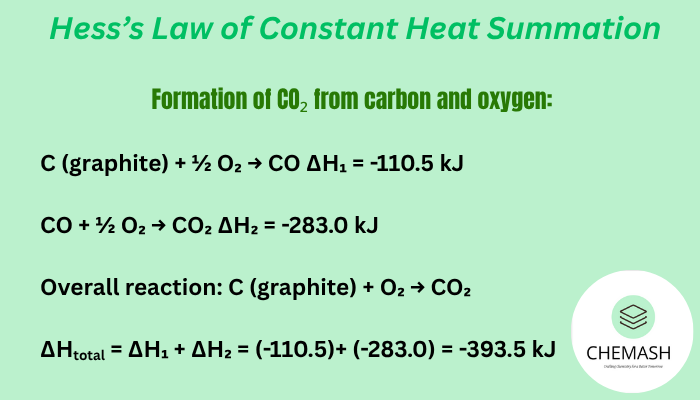
Example (CO₂ formation)
Formation of CO₂ from carbon and oxygen:
- C (graphite) + ½ O₂ → CO ΔH₁ = -110.5 kJ
- CO + ½ O₂ → CO₂ ΔH₂ = -283.0 kJ
Overall reaction: C (graphite) + O₂ → CO₂
ΔHtotal = ΔH₁ + ΔH₂ = (-110.5) + (-283.0) = -393.5 kJ
Importance and Applications
- Helps calculate enthalpy for reactions difficult to measure directly.
- Used in determining heats of formation and combustion.
- Forms the basis of Hess Cycles in thermochemistry.
- Useful in enthalpy and energy-efficiency studies in engineering and materials science.
Quiz
1. Hess’s Law is based on the fact that enthalpy is a:
- a) Path function
- b) State function ✅
- c) Variable function
- d) None
Explanation: Enthalpy depends only on initial and final states.
2. Total enthalpy change in a multi-step reaction is:
- a) Average of steps
- b) Sum of enthalpy changes ✅
- c) Zero
- d) Twice first step
Explanation: ΔH is additive for all steps.
Frequently Asked Questions
Q1: Why is Hess Law true?
A: Because enthalpy is a state function and independent of the reaction path.
Q2: What does Hess’s Law help calculate?
A: It helps determine enthalpy changes for reactions that are hard to measure experimentally.
Q3: Who discovered Hess Law?
A: Germain Henri Hess in 1840.
Up Next: Enthalpy — Understanding Heat Changes
