Hydrogen Bonding
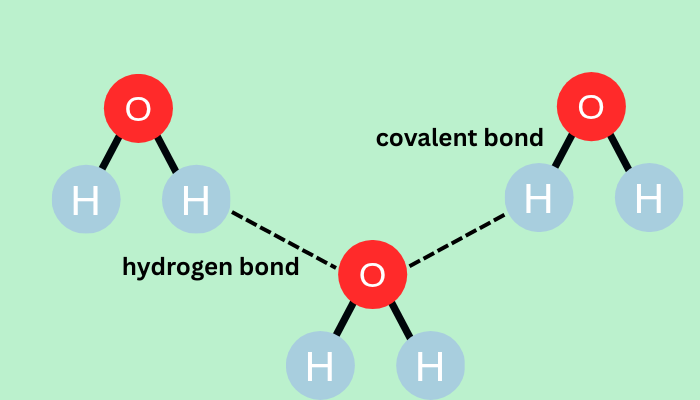
Hydrogen bonding is a special type of attractive interaction that occurs when a hydrogen atom covalently bonded to a highly electronegative atom (N, O, or F) experiences an attraction to another electronegative atom with a lone pair of electrons. This bond is weaker than covalent bonds but stronger than van der Waals forces.
Key Features
- Hydrogen Donor: Atom to which hydrogen is covalently bonded (N, O, or F).
- Hydrogen Acceptor: Electronegative atom with a lone pair that attracts hydrogen.
- Directional Bond: Hydrogen bonds are directional, usually linear.
- Strength: 5–30 kJ/mol (stronger than dipole interactions, weaker than covalent bonds).
Examples
- Water (H2O): Network of hydrogen bonds explains water’s high boiling point.
- Ammonia (NH3): Hydrogen bonds between N–H and lone pairs.
- Hydrogen Fluoride (HF): Very strong hydrogen bonding due to high electronegativity of F.
- Biological Molecules: DNA base pairing (A–T, G–C) and protein structures.
Effects
- Raises boiling and melting points.
- Explains water’s unique properties (surface tension, cohesion).
- Stabilizes biological macromolecules (DNA, proteins).
Summary Table
| Aspect | Details |
|---|---|
| Bond Type | Intermolecular or intramolecular dipole-dipole involving hydrogen |
| Atoms Involved | Hydrogen bonded to N, O, or F interacting with lone pairs on N, O, or F |
| Bond Strength | 5–30 kJ/mol |
| Importance | Water properties, DNA, protein stability |
MCQs
1. Which molecule shows the strongest hydrogen bonding?
✔ HF (due to highest electronegativity of F).
2. Which is NOT a hydrogen bonds donor?
✔ Carbon (C–H bonds rarely form strong H-bonds).
Quick Quiz
Why does water have an unusually high boiling point?
Because of extensive hydrogen bonding between water molecules.
What role does hydrogen bonding play in DNA?
Hydrogen bonds between base pairs stabilize the double helix structure.
Frequently Asked Questions
Q1: Is hydrogen bonding stronger than covalent bonding?
No, it is weaker than covalent but stronger than van der Waals forces.
Q2: Why is hydrogen bonding important in proteins?
It stabilizes secondary structures such as α-helices and β-sheets.
