Imperfections in Solids
Real crystals are never perfect. These irregularities, known as imperfections or crystal defects, significantly impact the physical and chemical properties of solids.
Types of Imperfections
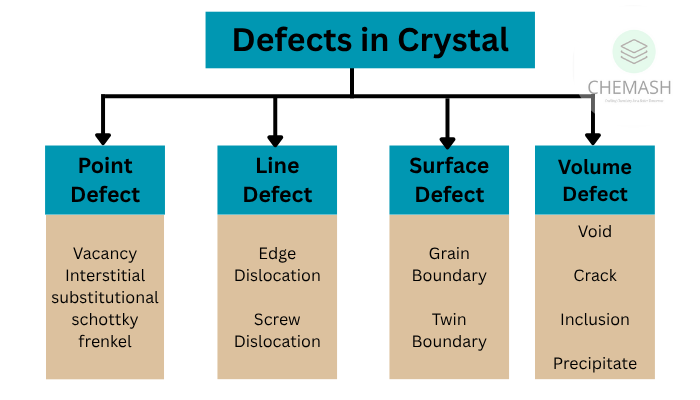
- Point Defects – At atomic scale
- Line Defects – Dislocations in a row
1. Point Defects
a. Stoichiometric Defects
- Vacancy: Missing atom
- Interstitial: Extra atom in space
- Frenkel: Ion leaves lattice and fits in a gap
- Schottky: Equal cations and anions missing
b. Non-Stoichiometric Defects
- Metal Excess: More cations/electrons than needed
- Metal Deficiency: Less cations than expected
c. Impurity Defects
Occurs when foreign ions are added (e.g., NaCl doped with SrCl₂).
2. Line Defects (Dislocations)
- Edge Dislocation: Extra half-plane of atoms
- Screw Dislocation: Spiral lattice twist
Importance
- Affect conductivity and strength
- Used in semiconductor doping
- Important in metallurgy and materials science
Conclusion: Crystal defects are crucial in determining real-world behavior of solids in chemistry, physics, and engineering.
MCQs: Test Your Understanding
- Which defect does not change the density of a crystal?
- A. Frenkel Defect ✅
- B. Schottky Defect
- C. Interstitial Defect
- D. Vacancy Defect
- Example of a compound showing Schottky defect is:
- A. ZnS
- B. NaCl ✅
- C. AgCl
- D. FeO
True or False (With Explanation)
- Frenkel defect increases the density of a crystal. ❌ False
Explanation: Frenkel defect doesn’t affect density as no ions are lost. - Schottky defect reduces the density of the solid. ✅ True
Explanation: Both cations and anions are missing, lowering mass per unit volume.

Pingback: Packing in Solids: Types, Efficiency, and Structures (SCP, BCC, FCC, HCP) -