Indicators are substances that exhibit different colors in acidic and basic media. They are crucial in identifying the chemical nature (acidic or basic) of a solution and are widely used in laboratories, titrations, and industries. Indicators and Their Uses
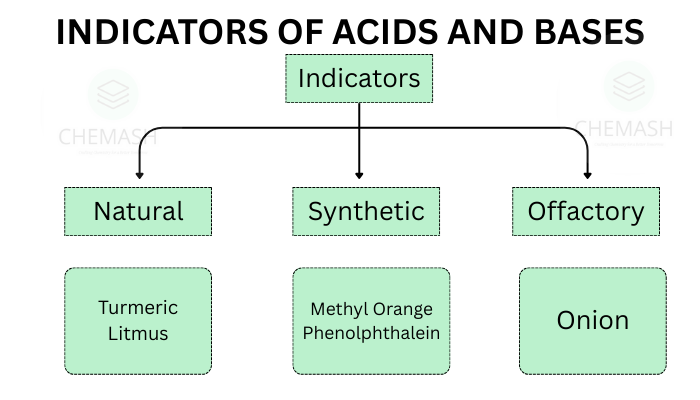
Types of Indicators
- Natural Indicators: Extracted from natural sources like plants. Common examples include litmus, turmeric, and red cabbage juice.
- Synthetic Indicators: Man-made indicators such as methyl orange and phenolphthalein used primarily in titrations.
- Olfactory Indicators: Change their odor in acidic or basic environments (e.g., onion, vanilla extract).
- Universal Indicators: A mixture of indicators that shows a gradual change in color over a range of pH values.
Common Acid-Base Indicators and Their Color Change
| Indicator | Color in Acid | Color in Base |
|---|---|---|
| Litmus | Red | Blue |
| Phenolphthalein | Colorless | Pink |
| Methyl Orange | Red | Yellow |
| Turmeric | Yellow | Red-Brown |
Uses of Indicators
- In Titration: To determine the end-point of acid-base neutralization reactions.
- In Laboratories: To test unknown substances and determine their pH range.
- In Education: Demonstrating acid-base reactions for academic purposes.
- In Industry: Used in pH-sensitive manufacturing processes, such as food production and pharmaceuticals.
- In Soil Testing: To check soil pH and guide agricultural practices.
- In Environmental Monitoring: Detecting acid rain or pollution through pH shifts.
Conclusion
Indicators are indispensable tools in identifying acidic and basic substances. Whether natural or synthetic, they provide visual cues that help chemists, students, and industries detect, analyze, and control pH-dependent reactions with high precision.
Quiz: Indicators and Their Uses
Select the best answer for each question and click Check Answers.
Q1: What color does phenolphthalein turn in a basic solution?
A) Red B) Yellow C) Colorless D) Pink
Q2: Which of the following is a natural indicator?
A) Methyl Orange B) Litmus C) Phenolphthalein D) Bromothymol Blue
Q3: What does turmeric turn in basic solution?
A) Yellow B) Blue C) Red-Brown D) Pink
Q4: Universal indicators are mainly used for:
A) Coloring solutions B) Narrow pH ranges C) Broad pH range detection D) Making compounds
Q5: What is the color of methyl orange in an acidic solution?
A) Yellow B) Red C) Orange D) Blue Check AnswersReset
FAQ
What is an indicator?
An indicator is a substance that changes color depending on the pH of the solution it is placed in. They are used to signal whether a solution is acidic or basic.
Which indicator is best for titrations?
Choice of indicator depends on the titration type and equivalence point pH. Phenolphthalein is commonly used for strong acid–strong base titrations with equivalence near pH 8–10; methyl orange is used when the equivalence is acidic.
Are natural indicators accurate?
Natural indicators like litmus or red cabbage juice are useful for demonstrations and rough pH estimates but are less precise than calibrated synthetic indicators or pH meters.
If you’d like these FAQs expanded into a dedicated FAQPage or translated into Hindi for bilingual content, I can add that too.
- External: Wikipedia — Indicator (chemistry)
Moreover, indicators play a vital role in analytical chemistry because they simplify the detection of end points. In addition, these indicators are widely used in industries such as food, pharmaceuticals, and water treatment. For example, phenolphthalein changes from colorless to pink in basic solutions, making it highly useful in titrations. On the other hand, methyl orange is more suitable for strong acid–weak base titrations due to its sharp and distinct color change. Therefore, choosing the correct indicator is crucial for obtaining accurate and reliable results. Consequently, a small mistake in selection can lead to incorrect interpretations of acidity or alkalinity. Furthermore, natural indicators like turmeric and red cabbage are eco-friendly alternatives that can be used in simple experiments. As a result, many educators prefer them for teaching students basic chemical concepts. Finally, indicators clearly demonstrate the importance of chemistry in both academic and industrial applications.
