Infrared Spectroscopy (IR) – Principle, Instrumentation, Interpretation & Applications
Infrared Spectroscopy (IR Spectroscopy) is one of the most important analytical techniques in chemistry used for the identification and structural analysis of chemical compounds. It is based on the interaction of infrared radiation with matter, resulting in absorption of energy due to vibrational transitions of molecules.
Table of Contents
- Introduction to Infrared Spectroscopy
- Principle of IR Spectroscopy
- Infrared Region of Electromagnetic Spectrum
- Molecular Vibrations
- IR Active and IR Inactive Molecules
- IR Spectrum Interpretation
- Important Functional Group Frequencies
- Instrumentation of IR Spectroscopy
- FTIR Spectroscopy
- Applications of IR Spectroscopy
- Limitations of IR Spectroscopy
- Frequently Asked Questions
Introduction to Infrared Spectroscopy
Infrared spectroscopy is a fundamental tool in physical, organic, inorganic and analytical chemistry. It provides valuable information about the presence of functional groups, molecular structure, bonding, and intermolecular interactions such as hydrogen bonding.
IR spectroscopy is extensively used in pharmaceutical industries, polymer science, environmental chemistry, forensic science and quality control laboratories. Because of its simplicity, non-destructive nature and high reliability, IR spectroscopy is often the first technique used for compound identification.
Principle of Infrared Spectroscopy
Atoms in a molecule are not stationary; they vibrate continuously around their equilibrium positions. When infrared radiation of appropriate frequency is passed through a molecule, absorption occurs if the frequency of radiation matches the natural vibrational frequency of the bond.
The absorbed energy causes a transition from a lower vibrational energy level to a higher vibrational energy level. The basic requirement for IR absorption is that the vibration must produce a change in dipole moment.
Mathematically:
ΔE = hν
Only those vibrations which involve a change in dipole moment are IR active.
Infrared Region of Electromagnetic Spectrum
| Region | Wavelength (µm) | Wavenumber (cm⁻¹) | Importance |
|---|---|---|---|
| Near Infrared | 0.78 – 2.5 | 14000 – 4000 | Overtones |
| Mid Infrared | 2.5 – 25 | 4000 – 400 | Structural analysis |
| Far Infrared | 25 – 1000 | 400 – 10 | Heavy atom vibrations |
Molecular Vibrations
Molecular vibrations are classified into stretching and bending vibrations. Stretching vibrations require more energy and therefore appear at higher wavenumbers than bending vibrations.
Stretching Vibrations
- Symmetrical stretching
- Asymmetrical stretching
Bending Vibrations
- Scissoring
- Rocking
- Wagging
- Twisting
IR Active and IR Inactive Molecules
For a molecule to be IR active, its vibration must cause a change in dipole moment. Homonuclear diatomic molecules such as H₂, N₂ and O₂ are IR inactive.
| Molecule | IR Activity | Reason |
|---|---|---|
| HCl | Active | Dipole moment present |
| CO₂ | Partially active | Asymmetric stretching |
| N₂ | Inactive | No dipole moment change |
IR Spectrum Interpretation
An IR spectrum is a plot of percent transmittance versus wavenumber. It is divided into two major regions: functional group region and fingerprint region.
Important Functional Group Frequencies
| Functional Group | Absorption Range (cm⁻¹) | Nature |
|---|---|---|
| O–H (Alcohol) | 3200 – 3600 | Broad |
| N–H | 3300 – 3500 | Medium |
| C=O | 1650 – 1750 | Strong and sharp |
| C≡N | 2210 – 2260 | Sharp |
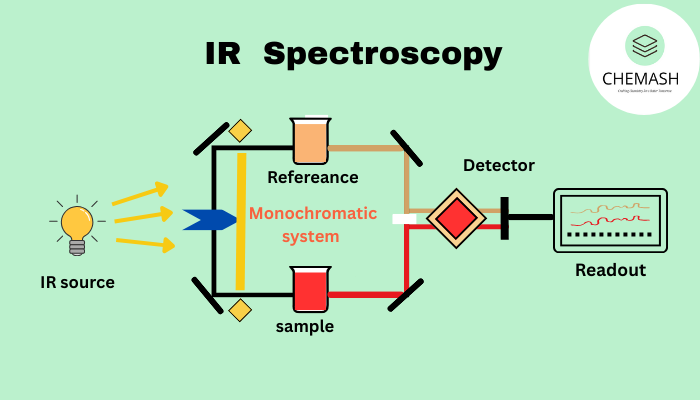
Instrumentation of IR Spectroscopy
A typical IR spectrometer consists of an infrared source, monochromator, sample holder, detector and recorder.
- Source: Nernst glower, Globar
- Monochromator: Prism or diffraction grating
- Sample handling: KBr pellet, NaCl plates
- Detector: Thermocouple, Bolometer
FTIR Spectroscopy
Fourier Transform Infrared Spectroscopy (FTIR) is an advanced form of IR spectroscopy that uses an interferometer instead of a monochromator. FTIR provides higher resolution, faster scanning and better signal-to-noise ratio.
Read more about UV Visible Spectroscopy on CHEMASH
Applications of IR Spectroscopy
- Identification of functional groups
- Pharmaceutical quality control
- Polymer characterization
- Environmental analysis
- Forensic investigations
Limitations of IR Spectroscopy
- Cannot analyze symmetric molecules
- Peak overlapping
- Limited quantitative capability
Frequently Asked Questions
What is Infrared Spectroscopy?
Infrared spectroscopy is an analytical technique used to study molecular vibrations caused by absorption of infrared radiation.
Why are homonuclear molecules IR inactive?
Because their vibrations do not produce a change in dipole moment.
What is fingerprint region?
The region between 1500–400 cm⁻¹ that is unique for each compound.
© CHEMASH – Chemistry Made Conceptual
