Introduction to Acids, Bases, and Salts
1. Acids
The study of acids, bases, and salts is fundamental in chemistry basics. These compounds are commonly found in nature, the human body, and in everyday materials. Moreover, they play crucial roles in industrial processes, biological functions, and environmental chemistry.Introduction to Acids, Bases, and Salts
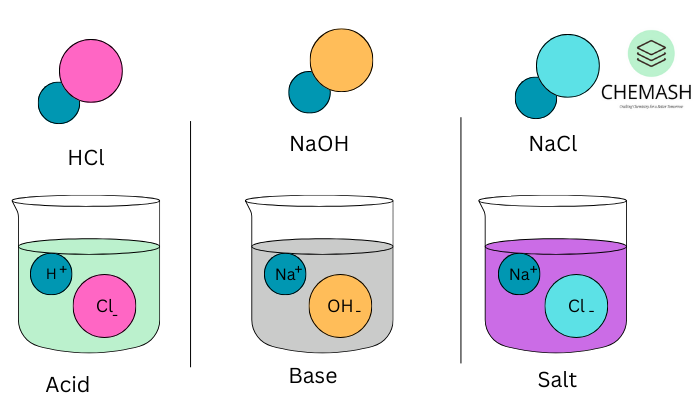
Acids are substances that release hydrogen ions (H⁺) in aqueous solutions. They generally have a sour taste and can turn blue litmus paper red. Furthermore, acids are corrosive and react vigorously with metals.
Examples: Hydrochloric acid (HCl), Sulfuric acid (H₂SO₄), Citric acid (C₆H₈O₇)
2. Bases
Bases are substances that release hydroxide ions (OH⁻) in aqueous solutions. In addition, they taste bitter, feel slippery, and turn red litmus paper blue. Strong bases can also be corrosive in nature.
Examples: Sodium hydroxide (NaOH), Ammonium hydroxide (NH₄OH), Magnesium hydroxide (Mg(OH)₂)
3. Salts
Salts are ionic compounds formed by the neutralization reaction between an acid and a base. They typically consist of the cation from the base and the anion from the acid.
Examples: Sodium chloride (NaCl), Potassium nitrate (KNO₃), Calcium sulfate (CaSO₄)
4. General Properties Comparison
| Property | Acids | Bases | Salts |
|---|---|---|---|
| Taste | Sour | Bitter | Varies |
| Litmus Test | Blue → Red | Red → Blue | No specific effect |
| Reaction with Metals | Produces H₂ gas | Generally no reaction | No direct reaction |
| pH Range | 0–6 | 8–14 | ~7 (neutral salts) |
5. Importance in Daily Life
- Acids: Used in car batteries (H₂SO₄), food (citric acid, vinegar), and digestion (HCl).
- Bases: Used in soaps, cleaning agents, and antacids.
- Salts: Used in food (NaCl), fertilizers (KNO₃), and construction (CaSO₄ in gypsum).
6. Neutralization Reaction
A neutralization reaction occurs when an acid and a base react to form salt and water. For example:
HCl + NaOH → NaCl + H₂O
7. Summary
In summary, acids, bases, and salts are vital chemical substances with unique properties. Acids release H⁺, bases release OH⁻, and salts result from their reaction. Therefore, understanding them is essential for exploring further chemical principles in chemistry and daily applications.
Quiz: Acids, Bases, and Salts
Q1: Which of the following is a property of acids?
A) Bitter taste
B) Turns blue litmus red
C) Produces OH⁻ in water
D) Slippery feel
Answer: B) Turns blue litmus red
Explanation: Acids turn blue litmus paper red due to hydrogen ion release.
Q2: A neutralization reaction produces:
A) Acid only
B) Base only
C) Salt and water
D) Hydrogen gas
Answer: C) Salt and water
Explanation: A reaction between acid and base yields salt and water.
Q3: Which of the following is a base?
A) HNO₃
B) CH₃COOH
C) NaOH
D) KCl
Answer: C) NaOH
Explanation: Sodium hydroxide is a strong base and releases OH⁻ in water.
Q4: What is the pH of a neutral salt solution usually?
A) Less than 7
B) 7
C) More than 7
D) It depends
Answer: B) 7
Explanation: Neutral salts like NaCl give a neutral solution with pH ~7.
A neutralization reaction occurs when an acid and a base react to form salt and water. For example:

Pingback: Types of Salts - CHEMASH
Acid base salt water lemon juice hydrogen peroxide oxygen