Introduction to Hydrocarbons
Hydrocarbons are the simplest organic compounds composed entirely of carbon (C) and hydrogen (H). They are the foundation of organic chemistry and form the basic framework for more complex organic molecules. Hydrocarbons are the major components of fuels such as petrol, diesel, LPG, and natural gas.
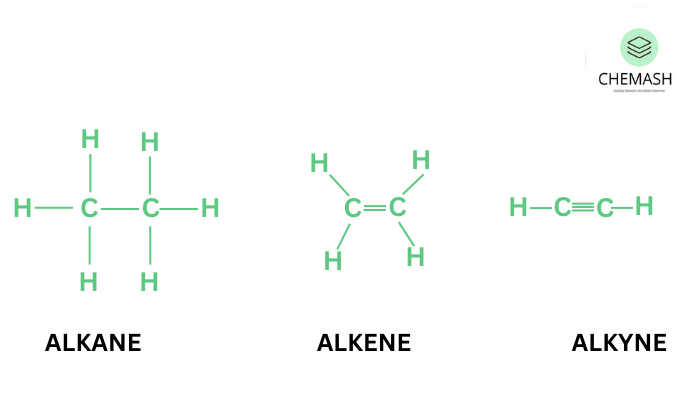
Classification of Hydrocarbons
- Saturated Hydrocarbons (Alkanes): Only single bonds (C–C). Formula: CnH2n+2.
- Unsaturated Hydrocarbons: At least one multiple bond.
- Alkenes: Double bond (C=C), formula: CnH2n.
- Alkynes: Triple bond (C≡C), formula: CnH2n−2.
- Aromatic Hydrocarbons: Contain benzene-like rings, show resonance, and follow Huckel’s rule.
Structure and Bonding
- σ-bonds (Sigma): Head-on overlap of orbitals (single bonds).
- π-bonds (Pi): Sidewise overlap of p-orbitals in double/triple bonds.
Sources of Hydrocarbons
- Natural Gas: Mainly methane (CH₄).
- Petroleum: Mixture refined into fuels & chemicals.
- Coal: Solid source rich in aromatics.
Importance of Hydrocarbons
- Used as fuels (LPG, petrol, diesel, CNG).
- Raw materials for plastics, detergents, pharmaceuticals.
- Backbone compounds in petrochemistry & organic synthesis.
Environmental Considerations
Burning hydrocarbons releases CO₂ (greenhouse gas). Incomplete combustion produces CO (carbon monoxide), which is toxic.
Fun Fact
The word hydrocarbon = hydrogen + carbon. From just two elements, we get millions of compounds!
MCQs on Hydrocarbons
- General formula of alkanes is:
a) CnH2n
b) CnH2n+2
c) CnH2n−2
Answer: (b) – Alkanes follow CnH2n+2. - Benzene is an example of:
a) Alkane
b) Alkyne
c) Aromatic hydrocarbon
Answer: (c) – Benzene is aromatic due to resonance.
Fill in the Blanks
1. General formula of alkynes is __________.
Answer: CnH2n−2.
2. Methane is the major component of __________.
Answer: Natural gas.
Quick Quiz
Q: Which type of hydrocarbon has only single bonds?
Ans: Alkanes (Saturated hydrocarbons).
FAQs on Hydrocarbons
Q1: What are hydrocarbons used for?
Ans: Mainly as fuels and raw materials in industries.
Q2: Which is the simplest hydrocarbon?
Ans: Methane (CH₄).
Q3: Why are hydrocarbons harmful to the environment?
Ans: Their combustion releases greenhouse gases and toxic CO.
Continue learning:
