Law of Chemical Equilibrium
The Law of Chemical Equilibrium (also called the Law of Mass Action) is a fundamental principle of chemistry that relates the concentrations of reactants and products in a reversible reaction at equilibrium. It is key to predicting the direction, yield, and balance of chemical reactions.
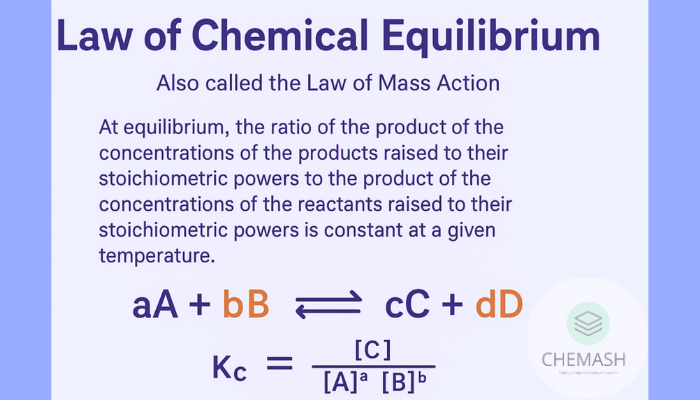
Statement of the Law
For a general reversible reaction:
aA + bB ⇌ cC + dD
where A and B are reactants, C and D are products, and a, b, c, d are stoichiometric coefficients, the law states:
At equilibrium, the ratio of the product of the concentrations of products raised to their stoichiometric powers to that of the reactants is constant at a given temperature.
Equilibrium Constant Expression
Mathematically, the equilibrium constant expression (Kc) is:
Kc = [C]c[D]d / [A]a[B]b
Here, square brackets [] denote molar concentrations at equilibrium.
Key Points
- Kc is constant only at a particular temperature.
- Only gases and aqueous species are included; solids and pure liquids are omitted.
- Large Kc (>103) → products favored.
- Small Kc (<10-3) → reactants favored.
Example
For the Haber process:
N2(g) + 3H2(g) ⇌ 2NH3(g)
The equilibrium expression is:
Kc = [NH3]2 / [N2][H2]3
Significance of the Law
- Predicts the direction of a reaction.
- Calculates equilibrium concentrations.
- Helps optimize industrial processes (e.g., Haber process).
- Explains reaction yields under different conditions.
Quiz: Test Your Understanding
- State the Law of Chemical Equilibrium in simple words.
- Write Kc for: 2SO2 + O2 ⇌ 2SO3.
- What does a large Kc value indicate?
- Why are solids excluded from Kc?
- How does temperature affect equilibrium constant?
Quiz Answers
- At equilibrium, product-to-reactant concentration ratio (with powers) is constant.
- Kc = [SO3]2 / [SO2]2[O2]
- Large Kc → product-favored.
- Because their concentrations remain constant.
- Kc changes as equilibrium shifts with temperature.
Multiple Choice Questions (MCQs)
- The Law of Chemical Equilibrium is also known as:
- a) Law of Conservation of Mass
- b) Law of Definite Proportions
- c) Law of Mass Action ✔
- d) Hess’s Law
- For H2 + I2 ⇌ 2HI, the expression is:
- a) [H2][I2] / [HI]2
- b) [HI]2 / [H2][I2] ✔
- c) [H2][HI] / [I2]
- d) [HI] / [H2]
- Which is NOT included in Kc?
- a) Gaseous reactants
- b) Aqueous ions
- c) Pure solids ✔
- d) Gaseous products
- A very small Kc (<1) indicates:
- a) Reaction favors products
- b) Reaction favors reactants ✔
- c) Reaction is complete
- d) Reaction is irreversible
FAQs on Law of Chemical Equilibrium
Q1. What is the Law of Chemical Equilibrium?
It states that at equilibrium, the ratio of product concentrations to reactant concentrations (raised to their stoichiometric powers) is constant at a given temperature.
Q2. Why are solids and liquids not included in Kc?
Because their concentrations remain constant and do not affect the equilibrium position.
Q3. What factors affect the equilibrium constant?
Only temperature. Pressure or concentration changes shift equilibrium but do not change Kc.
Q4. Where is this law applied in industry?
In processes like the Haber Process for ammonia synthesis and Contact Process for sulfuric acid production.
Q5. What does a large value of Kc signify?
It means the reaction strongly favors product formation at equilibrium.
