Magnetic Properties of Coordination Compounds
Magnetic properties of coordination compounds are an important aspect of inorganic chemistry because they reveal how unpaired electrons in the metal center influence the overall magnetic behavior of complexes. Depending on the electronic configuration, coordination compounds may show paramagnetism, diamagnetism, or even more complex magnetic interactions, making them essential in both theoretical studies and practical applications.
Table of Contents
Magnetic properties of coordination compounds arise primarily from the presence of unpaired electrons in the d-orbitals of the central metal ion. These properties give insights into bonding, geometry, and electronic structure. The type of magnetism depends on the number of unpaired electrons and ligand field strength.
1. Types of Magnetism
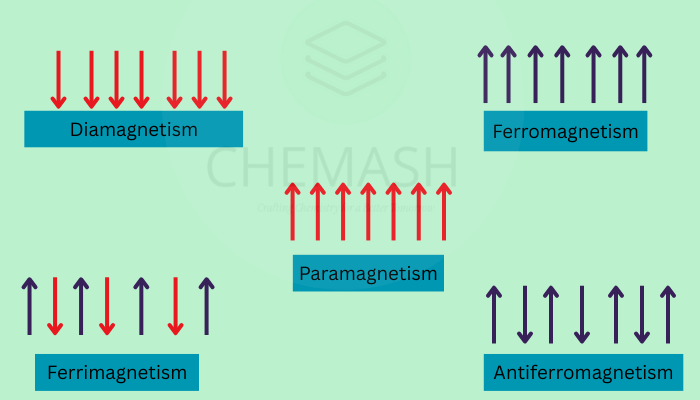
- Diamagnetism: All electrons paired, weakly repelled by a magnetic field.
- Paramagnetism: Presence of one or more unpaired electrons, attracted to a magnetic field.
- Ferromagnetism & Antiferromagnetism: Rare in complexes, caused by interactions between magnetic centers.
2. Origin of Magnetism
Depends on ligand field strength and electronic configuration. – Strong field ligands (CN⁻, NH₃) → low-spin, fewer unpaired electrons. – Weak field ligands (H₂O, F⁻) → high-spin, more unpaired electrons.
3. Magnetic Moment
The magnetic moment (μ) is measured in Bohr Magnetons (B.M.) and is calculated by the spin-only formula:
μ = √(n(n + 2)) B.M.
Where n = number of unpaired electrons.
4. Significance
- Helps determine electron configuration (high-spin vs low-spin).
- Provides insight into bonding type.
- Aids in predicting molecular geometry.
5. Examples
- [Fe(H₂O)₆]³⁺ → High-spin d⁵, five unpaired electrons, paramagnetic.
- [Fe(CN)₆]³⁻ → Low-spin d⁵, one unpaired electron, weakly paramagnetic.
- [Ni(CN)₄]²⁻ → Square planar, all electrons paired, diamagnetic.
Quiz: Test Your Knowledge
- What type of magnetism is exhibited by complexes with all paired electrons?
- How is magnetic moment related to unpaired electrons?
- What effect do strong field ligands have?
- Is [Ni(CN)₄]²⁻ paramagnetic or diamagnetic?
- Why are magnetic properties important in chemistry?
Answers: 1) Diamagnetism, 2) μ = √(n(n+2)), 3) They reduce unpaired electrons, 4) Diamagnetic, 5) For electron configuration and geometry.
Multiple Choice Questions (MCQs)
- The bond formed between a ligand and a metal ion is:
a) Ionic bond
b) Coordinate covalent bond
c) Metallic bond
d) Hydrogen bond - The formula for spin-only magnetic moment is:
a) μ = n(n + 1)
b) μ = √n
c) μ = √(n(n + 2))
d) μ = n + 2 - Strong field ligands usually form:
a) High-spin complexes
b) Low-spin complexes
c) Ionic bonds
d) Metallic bonds - The complex [Fe(H₂O)₆]³⁺ is:
a) Diamagnetic
b) Paramagnetic
c) Ferromagnetic
d) Antiferromagnetic - Diamagnetic substances:
a) Attracted to fields
b) Repelled by fields
c) Show spontaneous magnetization
d) Contain unpaired electrons
Frequently Asked Questions (FAQs)
Q1: What is the main cause of magnetism in coordination compounds?
A: The presence of unpaired d-electrons in the central metal ion.
Q2: Why is [Ni(CN)₄]²⁻ diamagnetic while [NiCl₄]²⁻ is paramagnetic?
A: CN⁻ is a strong field ligand causing pairing (low-spin), while Cl⁻ is weak field (high-spin).
Q3: What is the formula to calculate spin-only magnetic moment?
A: μ = √(n(n+2)) B.M., where n is the number of unpaired electrons.
Read more about Coordination Complexes and related bonding theories.
