Chemical Bonding – Metallic Bond
Metallic bonding is the type of chemical bonding that holds the atoms of a metal together. It explains characteristic metallic properties such as electrical conductivity, malleability, ductility, and luster. Unlike ionic or covalent bonding, metallic bonding is best understood using the electron sea model. Metallic Bond
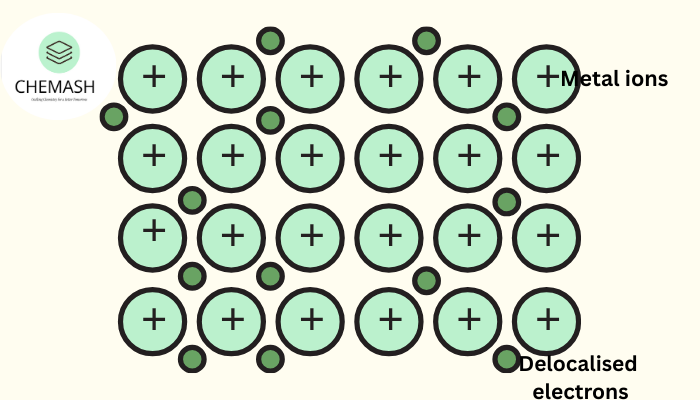
Electron Sea Model
In metallic bonding, metal atoms release some of their electrons, which become delocalized and move freely throughout the entire lattice. These electrons form a “sea of electrons” surrounding a lattice of positive metal ions.
- Electrons are not attached to specific atoms.
- They move freely, enabling conduction of electricity and heat.
- Electrostatic attraction between cations and electron cloud stabilizes the structure.
Key Properties of Metallic Bonding
- Electrical conductivity: Free electrons carry charge easily.
- Thermal conductivity: Heat spreads via mobile electrons and lattice vibrations.
- Malleability & ductility: Layers of atoms slide without breaking bonds.
- High melting/boiling points: Strong cation–electron attraction needs high energy to break.
- Luster: Free electrons reflect light, giving metals a shiny surface.
Factors Affecting Metallic Bond Strength
- Number of delocalized electrons: More electrons → stronger bonds.
- Cation charge: Higher positive charge → stronger attraction.
- Ion size: Smaller ions → stronger bonding due to closer packing.
Quiz: Test Your Understanding
- What is meant by the “sea of electrons”?
- Why do metals conduct electricity?
- What property allows metals to be hammered into thin sheets?
- Which three factors affect metallic bond strength?
- Why do metals have high melting points?
Answers
- It refers to delocalized electrons moving freely around the lattice.
- Because delocalized electrons carry charge across the metal.
- Due to malleability: layers slide without breaking metallic bonds.
- Number of delocalized electrons, cation charge, ion size.
- Strong attraction between ions and electron cloud requires large energy input.
FAQs on Metallic Bond
Q1: Is metallic bonding stronger than ionic bonding?
Ans: It depends; some metals (like W, Cr) have very strong metallic bonds, comparable to ionic/covalent bonds.
Q2: Why are alkali metals softer than transition metals?
Ans: Alkali metals have fewer delocalized electrons and larger atomic size, so bonds are weaker.
Q3: Where is metallic bonding applied in real life?
Ans: In electrical wiring, alloys (steel, brass), jewelry, and structural materials.
