Molecular Orbital Theory (MOT)
Molecular Orbital Theory (MOT) describes bonding by delocalized orbitals formed from the linear combination of atomic orbitals. It explains stability, magnetic properties, and bond order more accurately than valence bond theory.
Key Concepts
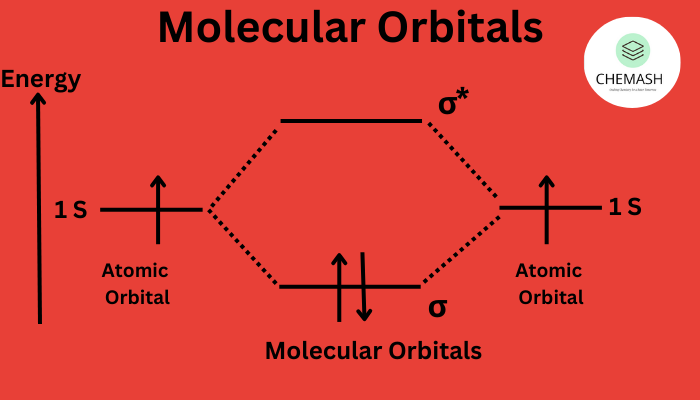
- Molecular orbitals form from the linear combination of atomic orbitals (LCAO).
- Bonding orbitals → lower energy; Antibonding orbitals (*) → higher energy.
- Total MOs = total combining AOs.
Types of Molecular Orbitals
- σ (Sigma): End-to-end overlap (s–s, s–pz, pz–pz).
- π (Pi): Sidewise overlap (px–px, py–py).
Bond Order
Bond Order = (Bonding Electrons − Antibonding Electrons) / 2
MO Energy Level Diagram
For B2, C2, N2 (Z ≤ 7):
σ1s < σ*1s < σ2s < σ*2s < π2p < σ2p < π*2p < σ*2p
For O2, F2, Ne2 (Z ≥ 8):
σ1s < σ*1s < σ2s < σ*2s < σ2p < π2p < π*2p < σ*2p
Examples
- H2: 2 e⁻ → Bond Order = 1 → Stable.
- He2: 4 e⁻ → Bond Order = 0 → Does not exist.
- O2: 16 e⁻ → Bond Order = 2 → Paramagnetic.
- N2: 14 e⁻ → Bond Order = 3 → Triple bond, very stable.
Important Points
- Explains paramagnetism (e.g., O2).
- Predicts bond order, bond length, and stability.
- Applicable to homoatomic and heteroatomic molecules (NO, CO).
MCQs (with Answers)
- Bond order of O2 is:
Ans: 2 (due to 16 electrons). - Which molecule is paramagnetic?
Ans: O2. - Why He2 does not exist?
Ans: Bond order = 0. - Bond order of N2 is:
Ans: 3.
FAQs
What is Molecular Orbital Theory?
MOT is a bonding theory where atomic orbitals combine to form delocalized molecular orbitals.
Why is O2 paramagnetic?
Oxygen has unpaired electrons in π*2p orbitals, making it paramagnetic.
Why does He2 not exist?
Bond order = 0, so it is unstable.
How is bond order calculated?
(Bonding e⁻ − Antibonding e⁻) / 2.
For further reading, check LibreTexts – Molecular Orbital Theory .
Molecular Orbital Theory
