Nernst Equation
Introduction
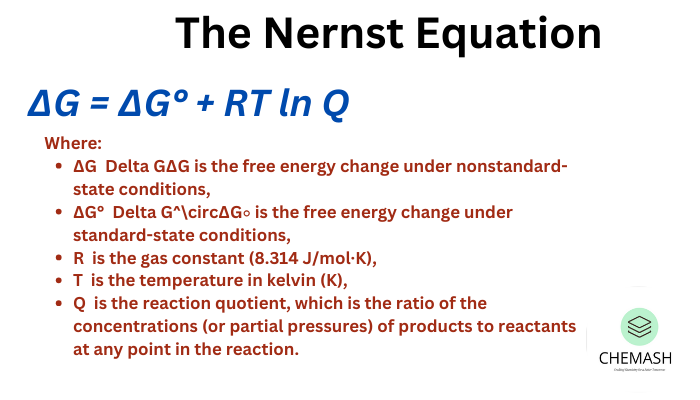
The Nernst equation relates the electrode potential under non-standard conditions to the standard electrode potential, temperature, and activities (or concentrations) of the involved species. It’s essential for predicting cell voltages in real systems.
Compute cell potentials under non-standard conditions — formula, variables, simplified form at 25°C, and applications.
Mathematical Form
For a general redox reaction: aA + ne⁻ → bB, the Nernst equation is:
E = E° − (RT / nF) · ln Q
Where:
- E = electrode potential (V)
- E° = standard electrode potential (V)
- R = 8.314 J·mol⁻¹·K⁻¹
- T = temperature (K)
- n = number of electrons transferred
- F = 96485 C·mol⁻¹ (Faraday constant)
- Q = reaction quotient
Simplified Nernst Equation at 25°C
At 298 K (25°C) the equation simplifies to:
E = E° − (0.0592 / n) · log₁₀ Q
Understanding the Terms
- Reaction quotient Q: Q = [Products]^b / [Reactants]^a (activities or concentrations).
- Concentration effect: Changing concentrations alters Q and therefore E.
- Temperature effect: Full equation shows explicit temperature dependence via RT/nF.
Applications
- Calculating cell potential under non-standard conditions.
- Determining equilibrium constants from cell potentials.
- Explaining pH meter (ion-selective electrode) response.
- Predicting how concentration changes affect battery voltage.
Quiz
- What does the Nernst equation calculate in an electrochemical cell?
- Write the simplified Nernst equation at 25°C.
- What is the significance of the reaction quotient Q?
- How does increasing product concentration affect the cell potential?
- Why is the Nernst equation important for pH meters?
Answers and Explanation
- It calculates the electrode potential (E) under non-standard conditions.
- E = E° − (0.0592 / n) log₁₀ Q
- Q reflects the instantaneous ratio of products to reactants and influences E.
- Increasing product concentration increases Q, which generally decreases E (for reductions written as products on top of Q).
- pH meters rely on the Nernst relationship between potential and hydrogen ion concentration to read pH from measured voltage.
FAQ
How do you calculate Q?
Q is calculated from current concentrations (or activities) as the product concentration terms divided by reactant concentration terms, each raised to their stoichiometric powers.
Does temperature make a big difference?
Yes — the full Nernst equation includes RT/nF, so temperature changes alter the slope; the simplified constant 0.0592 is valid only at 298 K.

Pingback: Electrode Potential - CHEMASH