Nitro Compounds (−NO₂) — Structure, Preparation, Properties & Uses
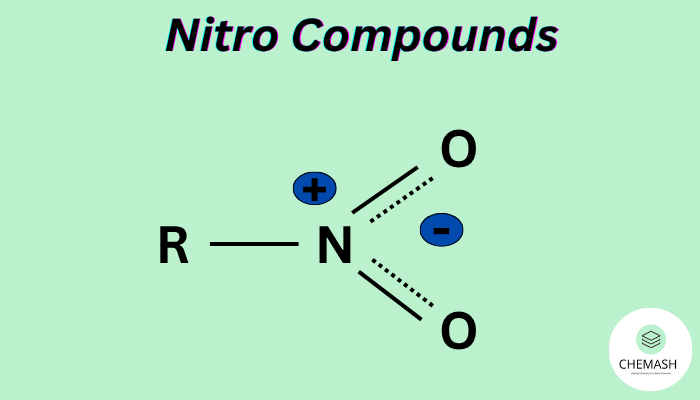
Definition: Nitro compounds are organic compounds containing one or more nitro groups (−NO₂) directly bonded to carbon atoms. The nitro group consists of one nitrogen atom doubly bonded to one oxygen and singly bonded to another oxygen atom bearing a negative charge (resonance structure).
Key Facts
- Functional group: −NO₂ (nitro group)
- General formula: R−NO₂
- Classification: Aliphatic & Aromatic nitro compounds
- Polarity: Strongly polar due to −NO₂ group
- Solubility: Soluble in organic solvents; some low molar mass nitro compounds dissolve in water
Structure of the Nitro Group
The nitro group has a planar structure with the nitrogen atom bonded to two oxygens (one via a double bond, one via a coordinate bond). The −NO₂ group shows resonance between structures:
R−N⁺(=O)−O⁻ ↔ R−N(−O⁻)=O⁺
This delocalization gives the nitro group stability and high electron-withdrawing character.
Classification
- Aliphatic Nitro Compounds: Nitroethane, nitromethane — used as solvents and fuel additives.
- Aromatic Nitro Compounds: Nitrobenzene, dinitrotoluene, trinitrotoluene (TNT) — used in dyes and explosives.
Preparation of Nitro Compounds
- Direct nitration: Aromatic hydrocarbons (e.g., benzene) react with concentrated HNO₃ and H₂SO₄ to yield nitrobenzene.
- Halide replacement: Alkyl halides react with silver nitrite (AgNO₂) to produce nitroalkanes.
- Oxidation: Amines can be oxidized to nitro compounds under controlled conditions.
Physical Properties
- High boiling points due to polarity.
- Moderate solubility in water; good solubility in alcohols and ethers.
- Yellow oily liquids or crystalline solids (depending on molecular size).
Chemical Properties & Reactions
- Reduction: Nitro compound can be reduced to amines using Sn/HCl, Fe/HCl, or catalytic hydrogenation.
- Nef reaction: Nitroalkanes on hydrolysis give carbonyl compounds.
- Acidic hydrogen: Nitroalkanes have α-hydrogen atoms that are slightly acidic due to the electron-withdrawing nitro group.
- Condensation reactions: Nitro compound participate in C–C bond-forming reactions (e.g., Henry reaction).
Uses of Nitro Compounds
- Manufacture of explosives like TNT (2,4,6-trinitrotoluene).
- Synthesis of dyes, perfumes, and pharmaceuticals.
- Solvents for cellulose and other organic compounds.
- Used as intermediates in amine synthesis.
Environmental and Biological Aspects
Some nitro compound are toxic and carcinogenic. Aromatic nitro compounds like nitrobenzene can affect the nervous system and must be handled carefully. However, in biology, controlled enzymatic nitro reduction is utilized in drug metabolism.
Practice MCQs
- MCQ 1: Nitrobenzene can be reduced to:
- A. Benzene
- B. Aniline
- C. Phenol
- D. Toluene
- MCQ 2: Which reagent is used in nitration of benzene?
- A. H₂O + H₂SO₄
- B. HNO₃ + H₂SO₄
- C. HCl + Zn
- D. NaOH + H₂O₂
- MCQ 3: Nitroalkanes have acidic hydrogen because:
- A. They contain −OH group
- B. The −NO₂ group is electron-withdrawing
- C. They contain double bonds
- D. They are aromatic
True / False
- Statement: Nitro compounds are weakly polar. — False. They are strongly polar due to the −NO₂ group.
- Statement: Nitrobenzene is used in making aniline. — True.
Frequently Asked Questions
Q: What is the difference between nitro and nitrate?
A: Nitro group (−NO₂) is a covalently bonded substituent on carbon; nitrate (NO₃⁻) is an ionic species.
Q: What happens when nitro compound are reduced?
A: They form amines, hydroxylamines, or azo compounds depending on reaction conditions.
Q: Why are aromatic nitro compound less reactive toward electrophilic substitution?
A: The −NO₂ group is strongly electron-withdrawing (−M and −I effects), deactivating the aromatic ring.
© 2025 CHEMASH — Educational Chemistry Content. Updated on November 3, 2025.

I regard something really special in this site.