Nomenclature of Coordination Compounds
The nomenclature of coordination compounds is a systematic way of naming complexes based on IUPAC rules. These rules ensure consistency and clarity in naming coordination chemistry structures worldwide.
Table of Contents
Basic Rules for Naming Coordination Compounds
- Name the ligands first: Alphabetical order, regardless of charge.
- Ligand names:
- Neutral ligands: ammine (NH₃), carbonyl (CO), aqua (H₂O)
- Anionic ligands: chloride → chloro, hydroxide → hydroxo, cyanide → cyano
- Polydentate: en (ethylenediamine), ox (oxalate)
- Number of ligands: mono-, di-, tri-, tetra-, penta-, hexa-. For complex ligands → bis-, tris-, tetrakis-.
- Name the central metal: – For cationic/neutral → cobalt, iron. – For anionic → ferrate, cuprate.
- Oxidation state: Shown in Roman numerals (III, II, IV).
- Ionic compounds: Name cation first, then anion.
Examples
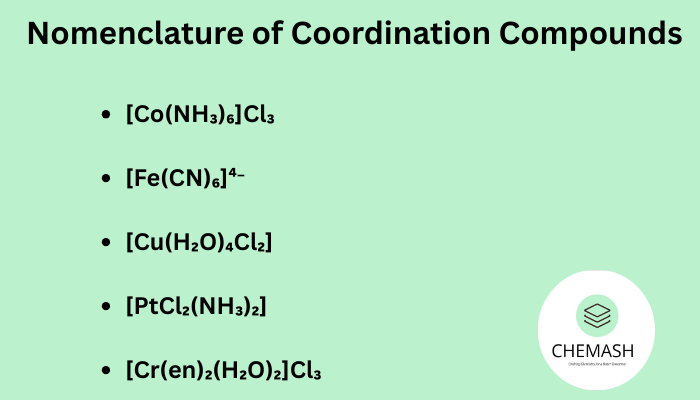
- [Co(NH₃)₆]Cl₃ → Hexaamminecobalt(III) chloride
- [Fe(CN)₆]⁴⁻ → Hexacyanoferrate(II) ion
- [Cu(H₂O)₄Cl₂] → Tetraaquadichlorocuprate(II)
- [PtCl₂(NH₃)₂] → Diamminedichloroplatinum(II)
- [Cr(en)₂(H₂O)₂]Cl₃ → Diethylenediaminediaquachromium(III) chloride
Special Notes
- Name ligands alphabetically (ignoring prefixes).
- Use bis-, tris-, tetrakis- for polydentate ligands.
- Anionic complexes → use suffix “-ate” (sometimes with Latin root: ferrum → ferrate).
Quiz: Test Your Understanding
- [Ni(CO)₄] → Tetracarbonylnickel(0)
- [Cr(H₂O)₆]Cl₃ → Hexaaquachromium(III) chloride
- Anionic complexes → metal ends in “-ate”.
- [Cu(en)₂Cl₂] → Bis(ethylenediamine)dichlorocopper(II)
- Two ethylenediamine ligands → Prefix “bis-”.
(MCQs)
- Suffix for metals in anionic complex?
a) -ium
b) -ate ✅
c) -ide
d) -ous - [Co(NH₃)₅Cl]²⁺ is named as:
a) Pentaamminochlorocobalt(II)
b) Pentaamminechlorocobalt(III) ✅
c) Pentaminechlorocobalt(III)
d) Pentamminochlorocobalt(III) - Ligand named “aqua”?
a) NH₃
b) H₂O ✅
c) CO
d) CN⁻ - Prefix for three ligands?
a) Tri-
b) Tetra-
c) Tris- ✅
d) Bis- - Oxidation state in [Fe(CN)₆]⁴⁻?
a) +2 ✅
b) +3
c) +4
d) +6
(FAQs)
Q1: What are neutral ligands called?
A: Neutral ligands retain their molecule names, e.g., NH₃ → ammine, H₂O → aqua.
Q2: How do we name anionic complexes?
A: Metals in anionic complexes end with “-ate”, e.g., [Fe(CN)₆]⁴⁻ → Hexacyanoferrate(II).
Q3: What is the role of oxidation states in nomenclature?
A: Oxidation states are shown in Roman numerals after the metal name.
