Nucleophilic Addition Reactions
Nucleophilic Addition Reactions are a major class of reactions in organic chemistry in which a nucleophile adds to a polar multiple bond, most commonly the carbon–oxygen double bond (C=O) present in aldehydes and ketones.
Definition
“A nucleophilic addition reaction is a reaction in which a nucleophile attacks an electron-deficient carbon atom of a polar double bond, resulting in the formation of an addition product.”
Why Carbonyl Compounds Undergo Nucleophilic Addition
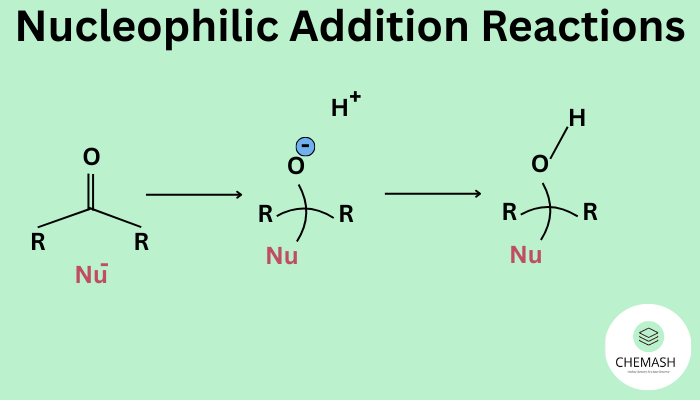
In aldehydes and ketones, the C=O bond is highly polar due to the electronegativity difference between carbon and oxygen.
- Carbon atom becomes electron-deficient (δ⁺)
- Oxygen atom becomes electron-rich (δ⁻)
- Nucleophiles attack the carbonyl carbon
General Mechanism of Nucleophilic Addition
- Nucleophilic attack: Nucleophile attacks the carbonyl carbon, breaking the π-bond.
- Formation of alkoxide ion: A tetrahedral intermediate is formed.
- Protonation: Alkoxide ion gains a proton to form the final product.
General Reaction
R–CHO / R–CO–R′ + Nu⁻ → R–C(OH)(Nu)–H / R–C(OH)(Nu)–R′
Important Examples of Nucleophilic Addition Reactions
1. Addition of Hydrogen Cyanide (HCN)
Aldehydes and ketones react with HCN to form cyanohydrins.
2. Addition of Sodium Bisulphite (NaHSO₃)
Aldehydes and ketones form bisulphite addition compounds, useful for purification and identification.
3. Addition of Grignard Reagents
Grignard reagents (RMgX) add to carbonyl compounds to form alcohols after hydrolysis.
4. Addition of Alcohols
Aldehydes and ketones react with alcohols in acidic medium to form hemiacetals and acetals.
Aldehydes vs Ketones (Reactivity)
| Aldehydes | Ketones |
|---|---|
| More reactive | Less reactive |
| Less steric hindrance | More steric hindrance |
| One alkyl group | Two alkyl groups |
Factors Affecting Nucleophilic Addition
- Nature of nucleophile
- Steric hindrance
- Electron-withdrawing or donating groups
- Reaction medium (acidic or basic)
Importance for Class 12 & NEET
Nucleophilic addition reactions are core reactions of aldehydes and ketones and are frequently asked in Class 12 board exams and NEET.
MCQs
Q1. Aldehydes are more reactive than ketones towards nucleophilic addition because:
- A. They have two alkyl groups
- B. They have less steric hindrance ✅
- C. They are non-polar
- D. They lack carbonyl group
Answer: B — Less steric hindrance and greater electrophilicity.
Frequently Asked Questions (FAQs)
Do carboxylic acids undergo nucleophilic addition?
No. Carboxylic acids generally undergo nucleophilic substitution instead of addition due to the presence of a good leaving group.
What type of intermediate is formed?
A tetrahedral intermediate is formed during nucleophilic addition.
