Oxidation Number
The oxidation number (oxidation state) is a bookkeeping tool to track electron transfer in redox chemistry.
Definition
The oxidation number of an atom is the hypothetical charge it would have if all bonds to atoms of different elements were 100% ionic. It is used to track electron transfer during oxidation–reduction (redox) reactions.
Rules for Assigning Oxidation Numbers
- Oxidation numbers of an element in its elemental form is 0 (e.g., O2, H2, Cl2).
- For a monoatomic ion, the oxidation numbers equals the ion charge (Na+ = +1).
- Fluorine is always -1 in compounds.
- Oxygen is usually -2, except in peroxides (-1), superoxides (-1/2), and when bonded to fluorine (+2).
- Hydrogen is +1 with non-metals and -1 with metals (e.g., NaH).
- The sum of oxidation numbers in a neutral molecule is 0; in a polyatomic ion it equals the ion’s charge.
Examples
- H2O → H = +1, O = -2 → 2(+1) + (-2) = 0
- NaCl → Na = +1, Cl = -1
- H2O2 → H = +1, O = -1 (peroxide)
- KMnO4 → K = +1, O = -2 (×4 = -8) → Mn = +7
- KMnO4 worked example: Sum = 0 → +1 + Mn + 4(-2) = 0 → Mn = +7.
Significance of Oxidation Number
- Identifies which elements are oxidized (increase in oxidation numbers) and reduced (decrease in oxidation numbers).
- Essential for balancing redox reactions using the oxidation numbers method.
- Used for electron bookkeeping in multi-step reaction mechanisms.
Quiz: Oxidation Numbers
Short Answer
- What is the oxidation number of oxygen in H2O2?
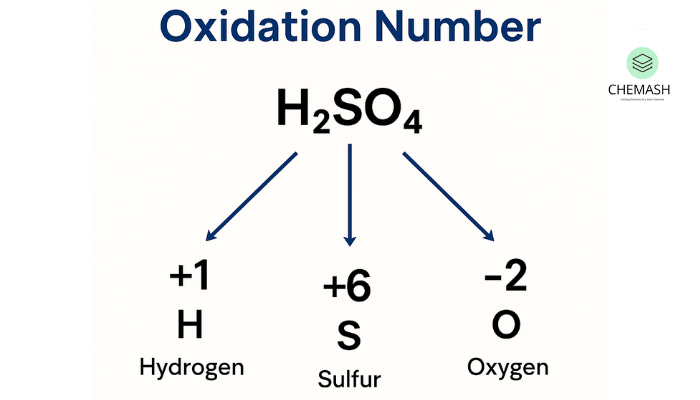
- Determine the oxidation number of sulfur in H2SO4.
- In which compound is hydrogen’s oxidation number -1?
- What is the oxidation numbers of Mn in MnO2?
- What is the oxidation numbers of Cl in Cl2O7?
Answers & Explanations
- Answer: -1. Explanation: In peroxides (H2O2) each O is -1.
- Answer: +6. Explanation: 2(+1) + S + 4(-2) = 0 → S = +6.
- Answer: In metal hydrides, e.g., NaH. Explanation: Hydrogen is -1 when bonded to metals.
- Answer: +4. Explanation: MnO2: 2(-2) = -4 → Mn = +4.
- Answer: +7. Explanation: Cl2O7: 7(-2) = -14 → total Cl = +14 → each Cl = +7.
MCQs (with brief explanations)
- Oxidation numbers of oxygen in most compounds is:
a) -1 b) -2 c) 0 d) +1 Explanation: Oxygen is usually -2 except in special cases like peroxides. - Oxidation number of hydrogen in NaH is:
a) +1 b) 0 c) -1 d) +2 Explanation: In metal hydrides hydrogen behaves as H–. - Oxidation number of chlorine in Cl2 is:
a) +1 b) -1 c) 0 d) +7 Explanation: In elemental diatomic chlorine the oxidation state is 0. - What is the oxidation number of Mn in KMnO4?
a) +4 b) +7 c) +6 d) +2 Explanation: K = +1, O = -8 total → Mn = +7 to balance to 0. - The oxidation number of sulfur in SO2 is:
a) +2 b) +4 c) +6 d) -2 Explanation: 2(-2) = -4 → S = +4 to make neutral molecule.
FAQ
What is the difference between oxidation numbers and oxidation state?
There is no practical difference—both terms are used interchangeably. They represent the hypothetical charge on an atom in a compound.
Why can oxygen have +2 in OF2?
Fluorine is more electronegative than oxygen and is assigned -1. To balance, oxygen becomes +2 in oxygen difluoride.
Can oxidation numbers be fractional?
Yes. For example in superoxides (O2–) each oxygen has oxidation number -1/2.
References & External Links
- LibreTexts — General Chemistry
- Wikipedia — Oxidation state (for quick reference)
- Related: Redox Reactions (CHEMASH)
- Contact CHEMASH
Published: September 28, 2025

Pingback: Oxidation and Reduction - CHEMASH