Phase Transitions
Phase transitions (solid ↔ liquid ↔ gas) explained: types, energy changes (endothermic/exothermic), latent heat, triple & critical points.
Introduction
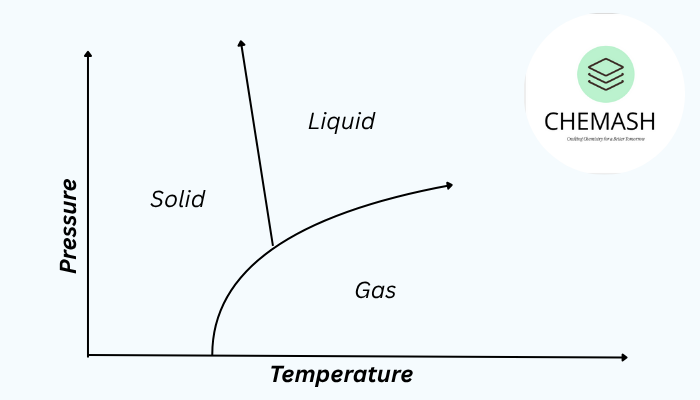
Phase transitions (or phase changes) are transformations of matter from one physical state to another — solid, liquid, gas, or plasma — caused by changes in temperature or pressure. These involve energy exchange, usually heat, and are described by thermodynamics and kinetics.
Types of Phase Transitions
- Melting (Fusion): Solid → Liquid (e.g., ice → water)
- Freezing: Liquid → Solid (e.g., water → ice)
- Boiling (Vaporization): Liquid → Gas (e.g., water → steam)
- Condensation: Gas → Liquid (e.g., steam → water)
- Sublimation: Solid → Gas (e.g., dry ice → CO₂ gas)
- Deposition: Gas → Solid (e.g., frost formation)
Energy Involved
Phase changes either absorb or release energy:
- Endothermic processes: Absorb heat — Melting, Boiling, Sublimation.
- Exothermic processes: Release heat — Freezing, Condensation, Deposition.
Important Concepts
- Latent Heat: Heat required to change the phase of a unit mass (e.g., latent heat of fusion, latent heat of vaporization) without changing temperature.
- Boiling Point: Temperature at which vapour pressure equals ambient pressure.
- Melting Point: Temperature at which solid becomes liquid.
- Triple Point: Specific temperature and pressure where solid, liquid and gas coexist in equilibrium.
- Critical Point: The highest temperature and pressure at which liquid and gas phases can coexist; above this a supercritical fluid forms.
Quiz: Phase Transitions
Short Answer
- What type of process is boiling?
- Name the transition from solid directly to gas.
- What is the triple point of a substance?
- Which phase change involves the release of energy?
- What happens to temperature during a phase change?
Answers & Explanations
- Answer: Endothermic. Explanation: Boiling requires input of heat to convert liquid to gas (latent heat of vaporization).
- Answer: Sublimation. Explanation: Solid directly converts to gas (e.g., dry ice).
- Answer: State where solid, liquid and gas coexist. Explanation: At specific T and P, three phases are in equilibrium (e.g., water at 0.01°C and 0.00604 atm).
- Answer: Freezing, condensation, deposition. Explanation: These processes release latent heat to the surroundings.
- Answer: It remains constant during the phase change. Explanation: Temperature does not change while the substance absorbs/releases latent heat to change phase.
MCQs (with brief explanations)
- Which phase transition is exothermic?
a) Melting b) Boiling c) Condensation d) Sublimation Explanation: Condensation releases heat as gas becomes liquid. - The temperature remains constant during:
a) Phase change b) Heating c) Cooling d) All of the above Explanation: During a phase change, added heat changes the phase (latent heat) not temperature. - Latent heat of fusion is the heat required for:
a) Gas → Liquid b) Solid → Liquid c) Liquid → Gas d) Liquid → Solid Explanation: Fusion is melting — solid to liquid. - Dry ice undergoes:
a) Condensation b) Sublimation c) Deposition d) Melting Explanation: Solid CO₂ converts directly to CO₂ gas at atmospheric pressure. - The critical point refers to:
a) Minimum temperature for boiling b) Point where solid melts c) Temperature beyond which gas can’t be liquefied d) Coexistence of all phases Explanation: Above critical T and P the distinction between liquid and gas disappears.
FAQ
What is latent heat?
Latent heat is the energy absorbed or released during a phase change per unit mass, without changing the temperature (e.g., latent heat of fusion for melting).
What is the triple point of water?
The triple point of water is at 0.01°C (273.16 K) and 611.657 Pa — temperature and pressure where solid, liquid and gas coexist in equilibrium.
Why does boiling point change with altitude?
Boiling point depends on ambient pressure. At higher altitudes atmospheric pressure is lower, so liquids boil at lower temperatures.
- LibreTexts — Thermodynamics & Phase Changes
- Wikipedia — Phase transition (overview)
- Related: Thermodynamics (CHEMASH)
Published: October 1, 2025
