Phosphate Group (-PO₄) — Structure, Properties, Functions & Biological Importance | CHEMASH
Published on November 2, 2025 · CHEMASH · Chemistry • Biochemistry
Phosphate Group (–PO₄) — Structure, Properties, Functions & Importance
Introduction
The phosphate group (–PO₄) is one of the most important functional groups in both chemistry and biology. It is derived from phosphoric acid (H₃PO₄) and plays key roles in energy metabolism (ATP), nucleic acids (DNA/RNA), and phospholipids.
Structure of the Phosphate Group
A phosphate group consists of one phosphorus atom covalently bonded to four oxygen atoms (–PO₄). Depending on protonation state, it can exist as H₂PO₄⁻, HPO₄²⁻, or PO₄³⁻.
- Chemical formula: –PO₄
- Charge: –1 to –3 depending on ionization
- Geometry: Tetrahedral around phosphorus
Physical & Chemical Properties
- Highly polar and hydrophilic due to oxygen atoms.
- Forms strong hydrogen bonds with water.
- Ionizable and contributes to acidity in molecules.
- Forms phosphate esters and anhydrides (e.g., ATP, ADP).
Formation & Reactions
Phosphate groups form ester linkages with alcohols (e.g., in nucleotides) and anhydride bonds in ATP. Hydrolysis of these bonds releases energy:
ATP → ADP + Pi + Energy
Phosphorylation (addition of a phosphate group) is catalyzed by kinases, while dephosphorylation (removal) is carried out by phosphatases.
Biological Importance
Phosphate groups are essential to life. They are found in:
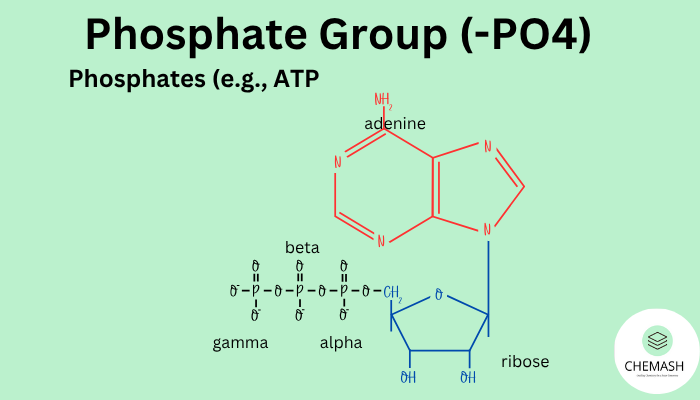
- ATP (Adenosine Triphosphate): Energy currency of the cell.
- DNA and RNA: Phosphate bridges link sugar molecules forming the backbone.
- Phospholipids: Major structural components of cell membranes.
- Protein Regulation: Phosphorylation controls enzyme activity and cell signaling.
Note: The reversible addition/removal of phosphate groups acts as a molecular switch in biological processes.
Chemical Importance
In inorganic chemistry, phosphate salts are used in fertilizers, buffers, and detergents. In organic chemistry, phosphates serve as protecting groups, catalysts, and intermediates in synthetic reactions.
Examples of Phosphate-containing Molecules
- ATP: Triphosphate molecule storing cellular energy.
- DNA: Sugar-phosphate backbone structure.
- Phospholipids: Amphipathic molecules forming membranes.
- Creatine phosphate: Energy reserve in muscles.
Quick MCQ Quiz (Test Your Knowledge)
- Which molecule contains multiple phosphate groups?
- A. Glucose
- B. ATP
- C. Ethanol
- D. Urea
- What is the geometry around the phosphorus atom in phosphate?
- A. Planar
- B. Linear
- C. Tetrahedral
- D. Trigonal bipyramidal
- Which enzyme adds phosphate groups to molecules?
- A. Hydrolase
- B. Kinase
- C. Dehydrogenase
- D. Synthase
- Phosphate groups in DNA connect:
- A. Bases to bases
- B. Sugars to phosphates
- C. Sugars to sugars
- D. Bases to sugars
Answer Key:
- B — ATP
- C — Tetrahedral
- B — Kinase
- C — Sugars to sugars (via phosphate bridge)
Frequently Asked Questions (FAQs)
Q: What charge does a phosphate group carry?
A: Typically –2 or –3 depending on the number of protons lost; it’s highly anionic and contributes to molecule polarity.
Q: What is phosphorylation?
A: The enzymatic addition of a phosphate group to an organic molecule (e.g., ADP → ATP), crucial in metabolism and signaling.
Q: How does ATP release energy?
A: Hydrolysis of the terminal phosphate bond in ATP releases free energy used by cells to drive biological processes.
© 2025 CHEMASH
