Published by CHEMASH — September 4, 2025
Polymers are large macromolecules formed by the repetitive bonding of small units called monomers. The process through which these monomers combine is called polymerization.
Definition
A polymer is a high molecular weight compound consisting of repeating structural units derived from monomers. They can be natural or synthetic and are widely used in daily life and industries.
Types of Polymers

- Based on Origin:
- Natural Polymers: Proteins, DNA, cellulose, rubber
- Synthetic Polymers: Nylon, polythene, PVC, Bakelite
- Based on Structure:
- Linear Polymers – e.g., High-density polyethylene (HDPE)
- Branched Chain Polymers – e.g., Low-density polyethylene (LDPE)
- Cross-linked Polymers – e.g., Bakelite, Melamine
- Based on Polymerization:
- Addition Polymers: Formed by addition reactions of alkenes and alkynes. Example: Polyethene from ethene.
- Condensation Polymers: Formed with the elimination of small molecules like water or HCl. Example: Nylon-6,6 from adipic acid and hexamethylene diamine.
Common Examples
- Polythene: Used in packaging and carry bags.
- Polyvinyl chloride (PVC): Used in pipes and electrical cable insulation.
- Nylon: Used in textiles and ropes.
- Bakelite: Used in electrical insulators and kitchenware.
- Teflon: Used for non-stick cookware and in high-performance materials.
Biodegradable Polymers
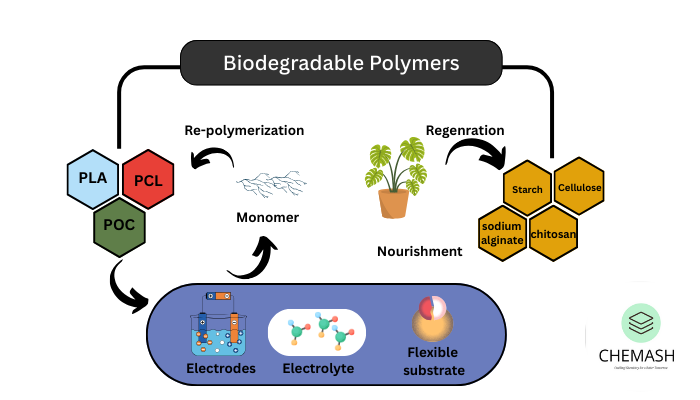
These polymers degrade in the environment by the action of microorganisms. Example: PHBV (Poly β-hydroxybutyrate-co-β-hydroxyvalerate).
Environmental Impact
Most synthetic polymers are non-biodegradable and contribute to environmental pollution. Development of green polymers and recycling techniques is essential.
Did You Know?
The word “polymer” comes from Greek, where “poly” means many and “meros” means parts — literally meaning “many parts”.
Related: Polymerization Methods .
External resources: Britannica — Polymers |
MCQs (Multiple Choice Questions)
- Which of the following is a condensation polymer?
- A. Polyethylene
- B. Nylon-6,6
- C. Polystyrene
- D. Polypropylene
- Which polymer is obtained by addition polymerization of ethene?
- A. Nylon
- B. Polyethylene
- C. Polyester
- D. Bakelite
- Which is a natural polymer?
- A. Teflon
- B. PVC
- C. Cellulose
- D. Nylon
- Cross-linked polymers typically exhibit:
- A. High solubility in water
- B. Thermoplastic behavior
- C. High dimensional stability and rigidity
- D. Low melting points
- Which term describes the repeating unit in a polymer chain?
- A. Monomer
- B. Oligomer
- C. Repeat unit (mer)
- D. Catalyst
(Click an MCQ answer in your CMS or use JS to reveal explanations when the student finishes.)
Quick Quiz — Self-check (5 points)
- Define polymer in one sentence. (write answer)
- Give two examples each of natural and synthetic polymers. (write answer)
- Explain the difference between addition and condensation polymerization. (write answer)
- Why are biodegradable polymers important? (write answer)
- Which type of polymer would you choose for making an insulating handle for a kettle — a cross-linked thermoset or a thermoplastic? Why? (write answer)
Scoring suggestion: award 1 point per clear, correct answer. 4–5 correct = Excellent, 2–3 = Good, <2 = Review topic.
Frequently Asked Questions (FAQ)
Q1: What is the difference between monomer and polymer?
A monomer is a small molecule that can join with other monomers. A polymer is a large molecule made by chemically bonding many monomers together.Q2: Are all plastics polymers?
Most plastics are polymers, though ‘plastic’ refers to a material category (mouldable synthetic polymers) that often contains additives, fillers and plasticizers.Q3: What makes a polymer biodegradable?
Biodegradability depends on chemical structure (e.g., hydrolyzable ester linkages), microbial action, and environmental conditions (temperature, moisture, microbes).Q4: What is the difference between thermoplastics and thermosets?
Thermoplastics soften on heating and can be remoulded; thermosets are cross-linked and do not melt — they degrade on heating instead of melting.Q5: How can we reduce polymer pollution?
Methods include recycling, using biodegradable polymers, redesigning products for less plastic, improved waste management, and circular economy strategies.
More topics: Applications of Polymers
