Preparation of Carboxylic Acids
Carboxylic acids (R–COOH) — common routes, examples, exam-ready MCQs, fill-ups and FAQs.
Table of Contents
Overview
Carboxylic acids are synthesized by several reliable transformations — oxidation, hydrolysis and carbon-chain building reactions. The choice depends on starting material, desired chain length and functional group tolerance.
Common Methods
1. From Primary Alcohols and Aldehydes (Oxidation)
Primary alcohols and aldehydes can be oxidized to carboxylic acids using strong oxidizing agents like potassium permanganate (KMnO4) or potassium dichromate (K2Cr2O7) under acidic or basic conditions.
Reaction:
R–CH2OH →[O] R–COOH
R–CHO →[O] R–COOH
- Example: Ethanol → Ethanoic acid (by strong oxidation).
- Widely used in lab and analysis.
2. From Alkylbenzenes (Side Chain Oxidation)
Side chains on benzene rings oxidize to benzoic acid derivatives regardless of chain length (as long as there is at least one benzylic hydrogen).
Reaction:
C6H5–CH3 →[KMnO4] C6H5–COOH
Used industrially to prepare benzoic acid and derivatives.
3. From Nitriles and Amides (Hydrolysis)
Nitriles (R–CN) and amides (R–CONH2) hydrolyse to carboxylic acids under acidic or basic hydrolysis with heat.
Reaction:
R–CN + 2 H2O →[H+/OH−, heat] R–COOH + NH3
R–CONH2 + H2O →[H+/OH−, heat] R–COOH + NH3
- Useful in organic synthesis for extending or transforming chains.
- Often used as a step after nucleophilic substitution (R–CN formation).
4. From Grignard Reagents
Grignard reagents react with CO2 to form carboxylate salts, which upon acidic work-up give carboxylic acids.
Reaction:
R–MgX + CO2 → R–COO−MgX+ →[H+] R–COOH
Valuable for synthesizing higher acids and for controlled chain-building.
5. From Carboxylic Acid Derivatives
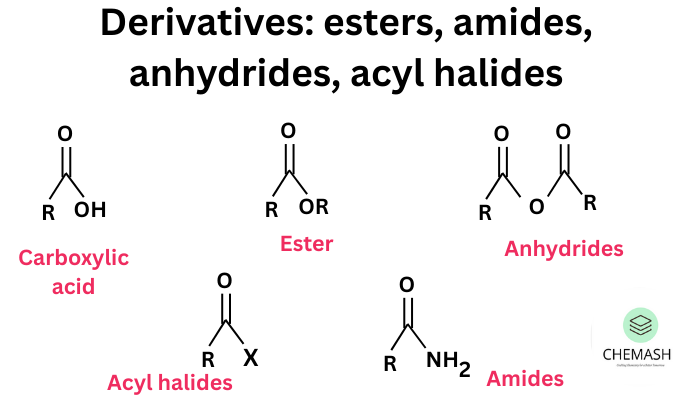
Acid chlorides, esters and anhydrides are hydrolysed to the parent carboxylic acids.
R–COCl + H2O → R–COOH + HCl
R–COOR′ + H2O →[H+/OH−] R–COOH + R′–OH
(RCO)2O + H2O → 2 R–COOH
6. From Trichloromethyl Ketones (Haloform Reaction)
Methyl ketones undergoing exhaustive halogenation followed by base cleavage produce carboxylates and haloform (CHX3), a diagnostic reaction.
Reaction:
CH3–CO–R + 3 X2 + 4 NaOH → R–COONa + CHX3 + 3 NaX + 2 H2O
Classic qualitative test for methyl ketones.
Conclusion
Summary: Carboxylic acids can be synthesized from alcohols, aldehydes, alkylbenzenes, nitriles, Grignard reagents, and acid derivatives. Choose the method based on starting material, desired product, and functional group compatibility.
MCQs (with answers & explanations)
Q1. Which reagent will oxidize ethanol to ethanoic acid under laboratory conditions?
Options: (A) PCC (B) KMnO4 (aq) (C) LiAlH4 (D) H2/Pd
Answer: (B) KMnO4
Explanation: KMnO4 (aqueous, strong oxidizer) converts primary alcohols fully to carboxylic acids. PCC oxidizes to aldehydes only; LiAlH4 reduces; H2/Pd reduces.
Q2. Oxidation of toluene (C6H5–CH3) with KMnO4 gives:
Options: (A) Phenol (B) Benzoic acid (C) Benzaldehyde (D) Benzyl alcohol
Answer: (B) Benzoic acid
Explanation: Side-chain oxidation of alkylbenzenes yields benzoic acid provided there is at least one benzylic hydrogen.
Q3. Grignard reagent R–MgBr treated with CO2 followed by H+ yields:
Options: (A) Alcohol (B) Ketone (C) Carboxylic acid (D) Ester
Answer: (C) Carboxylic acid
Explanation: The nucleophilic R– group attacks CO2 forming a carboxylate which gives the acid on acidification.
Fill in the blanks (with answers)
- Complete the reaction: R–CN + 2H2O → ______ + NH3.
Answer: R–COOH (carboxylic acid) - Grignard reagent reacts with ______ to give carboxylic acids after work-up.
Answer: CO2 - Haloform reaction generates a haloform (CHX3) and a ______ (salt) from methyl ketones.
Answer: carboxylate (e.g., R–COO−Na+)
Short Quiz — write answers below each (answers given)
Q1. What is the product when ethylbenzene is treated with hot KMnO4 and acidified?
Answer: Benzoic acid (C6H5COOH).
Why: Side-chain oxidation of ethylbenzene converts the alkyl side chain to a carboxyl group.
Q2. Why is PCC not suitable for converting ethanol to ethanoic acid?
Answer: PCC is a mild oxidant that stops at the aldehyde stage; strong oxidants like KMnO4 or K2Cr2O7 are needed to reach the carboxylic acid.
Frequently Asked Questions (FAQ)
Q: Can tertiary alcohols be oxidized to carboxylic acids?
A: No — tertiary alcohols lack the required hydrogen on the carbon bearing the –OH; they resist oxidation under normal conditions and often undergo C–C bond cleavage under very strong conditions.
Q: Which method is best if I want to extend a carbon chain by one carbon?
A: Use a Grignard reagent reacting with CO2 to add one carbon and form the carboxylic acid after acidic workup.
Q: Are there green alternatives to classic oxidants like KMnO4?
A: Yes—catalytic methods such as TEMPO-based oxidations or catalytic aerobic oxidations (using O2 with a catalyst) can be milder and greener depending on the substrate and scale. (For lab/industrial adoption evaluate cost & selectivity.)
If you want this as a bilingual (English + Hindi) page or need images/diagrams + inline MCQ scoring markup, tell me and I’ll generate the Hindi translation and image-ready SVGs for your post.
