Reaction Mechanisms – Complete Guide with Explanation, Steps, Examples & MCQs
Reaction Mechanism is the step-by-step sequence of elementary reactions by which an overall chemical reaction occurs. It explains how bonds break, how new bonds form, and why a reaction follows a particular pathway.
What is a Reaction Mechanism?
A reaction mechanism describes:
- The movement of electrons
- The formation of intermediates
- The breaking and making of chemical bonds
- The energy changes during a reaction
It is represented using curved arrow notation, which shows the flow of electrons.
Key Idea: Reaction mechanism explains the actual path taken by reactants to form products, not just the final equation.
Importance of Reaction Mechanisms
- Helps predict reaction products
- Explains reaction rate and selectivity
- Used in drug design and industrial synthesis
- Essential for understanding Organic Chemistry
Basic Components of Reaction Mechanism
| Component | Explanation |
|---|---|
| Reactants | Substances that take part in the reaction |
| Intermediates | Short-lived species formed during reaction |
| Transition State | High-energy unstable arrangement of atoms |
| Products | Final stable compounds formed |
Types of Reaction Mechanisms
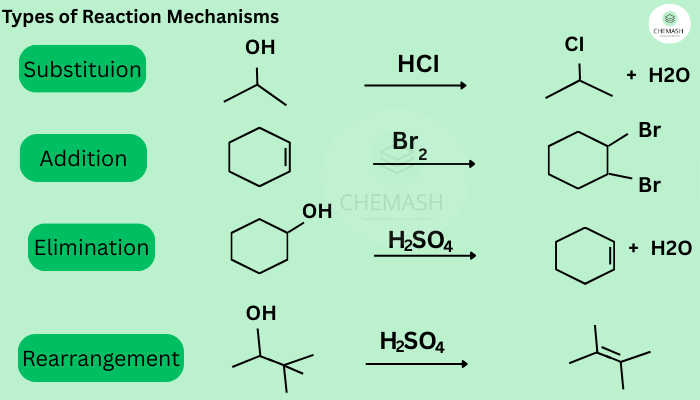
1. Substitution Reactions
One atom or group replaces another in a molecule.
- SN1 Mechanism – Two-step, carbocation intermediate
- SN2 Mechanism – One-step, backside attack
SN1 Mechanism (Example)
Hydrolysis of tertiary alkyl halide:
- Formation of carbocation (slow step)
- Nucleophile attack (fast step)
SN2 Mechanism (Example)
Reaction of methyl bromide with OH⁻ ion in one step.
2. Elimination Reactions
Removal of atoms or groups to form a double bond.
- E1 Mechanism – Two-step, carbocation intermediate
- E2 Mechanism – One-step, anti-periplanar geometry
3. Addition Reactions
Occurs mainly in alkenes and alkynes.
- Electrophilic addition
- Nucleophilic addition
- Free radical addition
Example: Addition of HBr to ethene.
4. Rearrangement Reactions
Atoms or groups shift within the molecule for stability.
- Hydride shift
- Alkyl shift
Curved Arrow Notation
Curved arrows represent the movement of electron pairs:
- Arrow tail → electron source
- Arrow head → electron destination
Note: Never move atoms, only electrons.
Energy Profile Diagram
An energy profile diagram shows:
- Activation energy
- Transition state
- Reaction intermediates
Lower activation energy = faster reaction.
Solved Example
Question: Why SN1 reactions show racemization?
Answer: SN1 reactions form a planar carbocation intermediate. The nucleophile can attack from both sides, producing a racemic mixture.
Frequently Asked Questions (FAQ)
Is reaction mechanism same as chemical equation?
No. Chemical equation shows only reactants and products, while reaction mechanism shows the complete pathway.
Why intermediates cannot be isolated?
They are highly unstable and exist for a very short time.
MCQs on Reaction Mechanism
- Which reaction proceeds through carbocation intermediate?
A. SN2
B. E2
C. SN1
D. Addition
Answer: C
Explanation: SN1 mechanism involves formation of carbocation. - SN2 reactions occur fastest with:
A. Tertiary alkyl halides
B. Secondary alkyl halides
C. Primary alkyl halides
D. Aromatic halides
Answer: C
Explanation: Less steric hindrance favors SN2. - Which arrow shows electron movement?
A. Straight arrow
B. Double arrow
C. Curved arrow
D. Dashed arrow
Answer: C
Explanation: Curved arrows represent electron flow.
Key Takeaways
- Reaction mechanism explains how reactions occur
- Curved arrows show electron movement
- Different mechanisms lead to different products
- Understanding mechanisms is essential for mastering Organic Chemistry
Khan Academy – Organic Chemistry

Pingback: Organic Chemistry याद करें NEET and Class 12 preparation strategy
Pingback: 12th Chemistry Board Exam Tips 2025 – Score 90+ Marks