Real Gases
Why some gases deviate from the ideal gas law (PV = nRT), how the Van der Waals equation corrects for intermolecular forces and finite volume, and how to use the compressibility factor Z to quantify deviations.
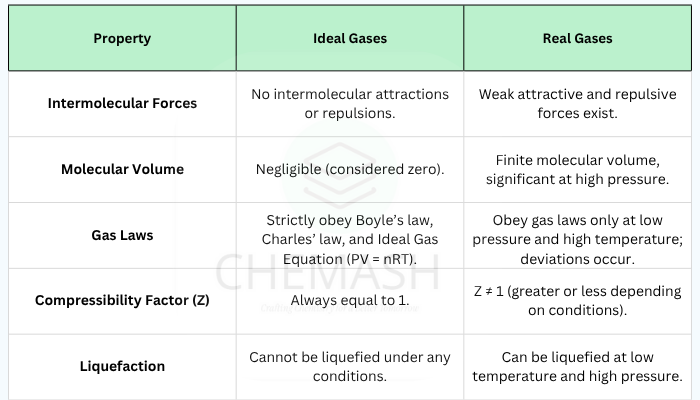
Ideal vs. Real Gases
| Property | Ideal Gas | Real Gas |
|---|---|---|
| Intermolecular Forces | Negligible | Present |
| Volume of Particles | Zero | Finite |
| Behavior at High Pressure | Follows ideal law | Deviates |
| Behavior at Low Temperature | Follows ideal law | Deviates |
Causes of Deviation
- Intermolecular attractions: Van der Waals forces reduce the effective pressure.
- Finite molecular volume: Molecules occupy space, reducing free volume.
Van der Waals Equation
The Van der Waals equation introduces two correction terms to the ideal gas law:
(P + a\frac{n^2}{V^2})(V - nb) = nRT
- a corrects for attractive forces (units vary with system).
- b corrects for finite molecular volume (exclusion volume).
Use Van der Waals to get better predictions for P–V–T behaviour near condensation or at high pressures.
Compressibility Factor (Z)
The compressibility factor quantifies deviation from ideality:
Z = \dfrac{PV}{nRT}
- If Z = 1 → Ideal behaviour.
- If Z < 1 → Attractive forces dominate (observed at intermediate pressures).
- If Z > 1 → Repulsive forces dominate (observed at very high pressures).
Conditions for Ideal and Real Behavior
- Gases behave ideally at high temperatures and low pressures.
- Gases behave non‑ideally at low temperatures and high pressures.
- Lighter gases (He, H2) are closer to ideal than heavier gases (CO2, SO2).
Quiz: Real Gases
Short Answer
- What causes deviation of real gases from ideal behavior?
- What is the Van der Waals equation used for?
- When is the compressibility factor Z equal to 1?
- Which type of gases behave more ideally—lighter or heavier?
- How does low temperature affect gas behavior?
Answers & Explanations
- Answer: Intermolecular forces and finite molecular volume. Explanation: These factors cause P and V to deviate from ideal predictions.
- Answer: To correct the ideal gas law for intermolecular forces and finite molecular sizes. Explanation: The a and b parameters improve accuracy near condensation and at high P.
- Answer: When the gas behaves ideally. Explanation: Z = 1 means PV = nRT holds for that state point.
- Answer: Lighter gases (e.g., He). Explanation: Smaller molecules with weaker interactions approximate the ideal assumptions better.
- Answer: Gases deviate more from ideal behavior (intermolecular attractions stronger, condensation possible). Explanation: Lower T increases relative importance of attractive forces.
MCQs (with brief explanations)
- Which constant in the Van der Waals equation corrects for attractive forces?
a) b b) a c) R d) n Explanation: ‘a’ accounts for intermolecular attractions, lowering pressure. - Real gases deviate most from ideal behavior at:
a) High temp, low pressure b) Low temp, high pressure c) Low temp, low pressure d) High temp, high pressure Explanation: Low T and high P increase interactions and molecular volume effects. - For a gas with Z < 1, the dominant force is:
a) Attractive b) Repulsive c) Zero d) None Explanation: Z < 1 indicates measured PV is less than ideal — attractions reduce pressure. - The value of Z for an ideal gas is:
a) 0 b) 1 c) Greater than 1 d) Less than 1 Explanation: Ideal gas obeys PV = nRT so Z = 1. - Which gas is most likely to behave ideally?
a) CO2 b) NH3 c) He d) SO2 Explanation: Helium is small, monatomic and weakly interacting — closest to ideal.
FAQ
Why does the ideal gas law fail at high pressure?
At high pressure the volume occupied by gas molecules is no longer negligible compared to the container volume, and interparticle repulsions become significant; both effects violate ideal assumptions.
How do you experimentally determine the compressibility factor Z?
Measure P, V, T and amount n for a gas sample and compute Z = PV / (nRT). Tabulated Z vs reduced pressure/temperature (or charts) are often used.
Are Van der Waals constants universal?
No — ‘a’ and ‘b’ are specific to each gas and are determined experimentally or from more detailed molecular models.
- LibreTexts — Gases and Real Gas Behaviour
- Wikipedia — Van der Waals equation
- Related: Gaseous State (CHEMASH)
Published: October 1, 2025

Pingback: Ideal Gas Law - CHEMASH