Solid State Chemistry
Solids form one of the fundamental states of matter because their particles are closely packed and held together by strong intermolecular forces. Consequently, unlike gases and liquids, solids maintain a definite shape and volume due to restricted particle movement. Solid State Chemistry
Classification of Solids
Solids are classified based on the type of bonding between their constituent particles. Specifically, they are divided into:
- Crystalline Solids: These solids have a well-ordered, repeating arrangement of atoms, ions, or molecules.
- Amorphous Solids: In contrast, these solids lack long-range order and are also called pseudo-solids or supercooled liquids. For example, glass.
Types of Crystalline Solids
Crystalline solids can be further categorized based on their constituents and bonding forces:
| Type | Constituents | Forces | Examples |
|---|---|---|---|
| Ionic | Ions | Electrostatic | NaCl, MgO |
| Molecular | Molecules | Van der Waals / Hydrogen bonds | Ice, CO2 |
| Covalent (Network) | Atoms | Covalent bonds | Diamond, SiO2 |
| Metallic | Positive ions in electron sea | Metallic bonds | Cu, Fe |
Properties of Solids
- Definite shape and volume: Particles are fixed in a rigid structure, therefore, solids maintain their shape.
- High density: Since particles are closely packed, solids have higher densities than liquids or gases.
- Incompressibility: Due to negligible intermolecular spaces, solids resist compression.
- Melting Point: Crystalline solids exhibit sharp melting points, whereas amorphous solids gradually soften upon heating.
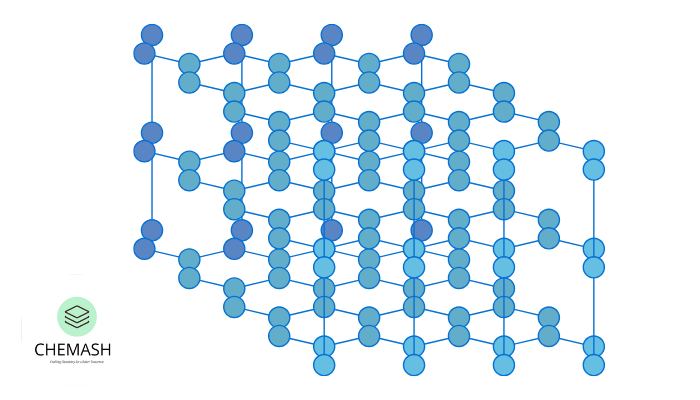
Crystal Lattices and Unit Cells
Crystalline solids consist of unit cells, which are the smallest repeating structural units. Moreover, the three-dimensional arrangement of these unit cells forms a lattice.
There exist 7 crystal systems and 14 Bravais lattices, which describe all possible unit cell types, including cubic and tetragonal structures.
Defects in Solids
Imperfections in solids are called defects. In particular, we can classify them as follows:
- Point Defects: Vacancies, interstitials, and substitutional defects.
- Stoichiometric Defects: Schottky and Frenkel defects that preserve the overall ratio of ions.
- Non-Stoichiometric Defects: These defects alter the stoichiometric ratio, often due to extra cations or anions.
Electrical Properties
Certain solids can conduct electricity. For instance:
- Conductors: Metals contain free electrons that allow efficient conduction.
- Insulators: Non-metals lack free charge carriers, therefore they do not conduct.
- Semiconductors: Silicon and Germanium exhibit controllable conductivity, which can be enhanced with doping.
Quiz – Test Your Knowledge
- Which of the following is an example of an amorphous solid?
a) Diamond
b) Quartz
c) Glass
d) Sodium chloride
Answer: c)
Explanation: Glass lacks long-range order, making it an amorphous solid. - In which type of solid are positive ions immersed in a sea of electrons?
a) Ionic
b) Metallic
c) Covalent
d) Molecular
Answer: b)
Explanation: Metallic solids consist of positive metal ions surrounded by mobile electrons. - Which defect maintains electrical neutrality but reduces density?
a) Frenkel defect
b) Schottky defect
c) Substitutional defect
d) Interstitial defect
Answer: b)
Explanation: Schottky defect involves the loss of equal numbers of cations and anions, reducing density.
Next Topic: Crystal Lattice and Unit Cell Geometry
