Published by CHEMASH • Updated for clarity • September 6, 2025.
Overview
Stereochemistry studies how the 3-dimensional arrangement of atoms affects chemical and physical properties. It’s essential for understanding reaction mechanisms, biological activity of drugs, and material behaviour.
Example: Enantiomers of drugs (e.g., ibuprofen) can have different biological effects.
Types of Isomerism
- Structural (Constitutional) Isomerism – different connectivity of atoms.
- Stereoisomerism – same connectivity but different 3D arrangement.
Stereoisomerism Classification
- Conformational Isomerism – rotation around single bonds (Newman).
- Configurational Isomerism – cannot interconvert by rotation alone; includes geometrical (cis/trans, E/Z) and optical (chiral) isomerism.
Geometrical Isomerism (cis-trans / E-Z)
Occurs where rotation is restricted (e.g., C=C double bonds, small rings).
- Cis – identical groups on the same side.
- Trans – identical groups on opposite sides.
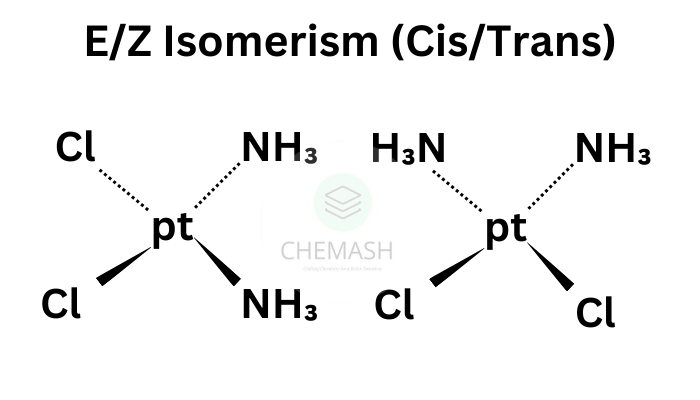
E–Z System (Cahn–Ingold–Prelog)
Used when substituents are different; assign priority by atomic number and compare positions.
Example: 2-butene — cis (Z): CH3 groups same side; trans (E): opposite sides.
Optical Isomerism & Chirality
Enantiomers are non-superimposable mirror images. A molecule is chiral if it has no internal plane of symmetry.
- Chiral center: typically a carbon attached to four different groups.
- Enantiomers: identical physical properties except rotation of plane-polarized light and interactions with other chiral substances.
- Diastereomers: stereoisomers that are not mirror images.
Optical Activity
Measured with a polarimeter: dextrorotatory (+) rotates clockwise; levorotatory (−) rotates anticlockwise.
Example: Lactic acid (CH3–CH(OH)–COOH) has one chiral center → two enantiomers.
Meso Compounds
A meso compound has multiple stereocentres but an internal symmetry plane that makes it achiral.
Example: Tartaric acid (one stereochemical form is meso and optically inactive).
Representation of Stereochemistry
- Fischer projections — convenient for sugars and amino acids.
- Newman projections — visualize conformations around single bonds.
- Sawhorse projections — diagonal 3D perspective.
- Wedge–dash notation — solid wedge out of page, hashed wedge into page.
Importance of Stereochemistry
- Drug design and pharmacology
- Enzyme specificity
- Flavors and fragrances
- Polymer properties and materials science
Interesting fact: The thalidomide tragedy (1960s) highlighted the importance of stereochemistry — one enantiomer caused birth defects.
MCQs
- Which of the following defines a chiral carbon?
- a) Carbon with three identical groups
- b) Carbon with four different groups
- c) Carbon with a double bond
- d) Carbon in a ring
Explanation: A chiral center (stereocenter) usually has four different substituents, creating non-superimposable mirror images. - Which isomerism is due to restricted rotation about a bond?
- a) Optical isomerism
- b) Structural isomerism
- c) Geometrical (cis–trans) isomerism
- d) Conformational isomerism
Explanation: Restricted rotation, such as around a double bond, gives rise to cis/trans or E/Z isomers. - Which projection is best for showing staggered and eclipsed conformations?
- a) Fischer
- b) Newman
- c) Wedge–dash
- d) Sawhorse
Explanation: Newman projections show view down a bond and can represent staggered/eclipsed conformations clearly. - Which system assigns priority when naming E/Z isomers?
- a) R/S rules
- b) Cahn–Ingold–Prelog priority rules
- c) Fisher projection rules
- d) Markovnikov’s rule
Explanation: CIP rules assign priorities to substituents to determine E or Z configuration.
Quiz (10 questions with answers)
- What is a chiral carbon? A carbon atom with four different groups attached.
- Which type of isomerism arises due to restricted rotation? Geometrical (cis–trans) isomerism.
- What is the difference between enantiomers and diastereomers? Enantiomers are mirror images; diastereomers are not mirror images and have different physical properties.
- Which system is used to assign priority in E/Z isomerism? Cahn–Ingold–Prelog rules.
- What is a meso compound? A molecule with multiple stereocentres but an internal plane of symmetry making it achiral.
- Name two projection methods used in stereochemistry. Fischer and Newman projections.
- What property is used to distinguish enantiomers in the lab? Optical rotation of plane-polarized light measured with a polarimeter.
- What type of isomers are cis- and trans-butene? Geometrical isomers (E/Z isomers).
- What causes optical activity in a molecule? Presence of chirality (asymmetric centers) or overall chiral arrangement.
- Which projection shows the conformation of single bonds? Newman projection.
Frequently Asked Questions
Q: How do I determine if a molecule is chiral?
A: Look for stereocentres (atoms with four different substituents) and absence of an internal plane of symmetry. Try to draw its mirror image — if non-superimposable, it’s chiral. Q: Can a molecule with two chiral centres be achiral? A: Yes — if the molecule has an internal plane of symmetry (a meso compound), it can be achiral despite stereocentres. Q: What’s the practical importance of stereochemistry in drugs? A: Enantiomers can interact differently with biological targets; one enantiomer could be therapeutic while the other is inactive or harmful. Drug development often isolates the active enantiomer
References & Links
- External: Stereochemistry — Wikipedia
